Flavonoid derivative for treating osteoporosis as well as pharmaceutical composition and application thereof
A technology of osteoporosis and flavonoids, applied in the field of medicine, can solve the problems of increasing human and financial burden on families and society
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Synthesis of 3'-chloro-7-ethoxyisoflavones.
[0076] The synthesis steps are:
[0077]
[0078] ;
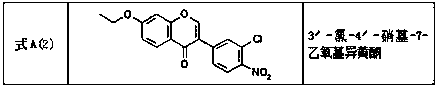
[0079] Dissolve 4-ethoxy-2-hydroxyacetophenone B (20g, 6.5mol) in tetrahydrofuran (100ml), add lithium hexamethyldisilazide (LiHMDS) (60ml), stir and mix for 2h to ensure that the acyl Lithium enolate can be completely formed, then add 3-chlorobenzoyl chloride C (22g, 6.5mol) and continue stirring for 3h to generate β-propanedione compound D; add sulfuric acid (30ml) and Acetic acid (20ml), heated at 120°C and refluxed for 4-5h, initially cooled to 22°C, adjusted the pH to 8 with an alkali neutralizer, and solids precipitated; continued to cool the precipitated solids to 5°C, stirred and crystallized for 1h, filtered, and used The filter cake was washed with ethyl acetate, followed by recrystallization with methanol, acetone and pure water, and vacuum-dried to obtain solid compound 3'-chloro-7-ethoxyisoflavone A (1) (37.2g), 94.5%.
Embodiment 2
[0081] Synthesis of 3'-chloro-4'-nitro-7-ethoxyisoflavones.
[0082] The synthesis steps are:
[0083]
[0084] ;
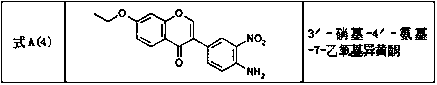
[0085] Dissolve 4-ethoxy-2-hydroxyacetophenone B (20g, 6.5mol) in tetrahydrofuran (100ml), add lithium hexamethyldisilazide (LiHMDS) (60ml), stir and mix for 2h to ensure that the acyl Lithium enolate can be completely formed, then add 3-chloro-4-nitrobenzoyl chloride C (22g, 6.5mol) and continue to stir for 3h to generate β-propanedione compound D; add to the reaction system Sulfuric acid (30ml) and acetic acid (20ml), heat at 100°C and reflux for 4-5h, initially cool down to 20°C, adjust the pH to 9 with an alkali neutralizer, and solids precipitate; continue to cool the precipitated solids to 10°C, stir and crystallize Filter after 1h, wash the filter cake with ethyl acetate, recrystallize with methanol, acetone and pure water successively, and dry in vacuo to obtain the solid compound 3'-chloro-4'-nitro-7-ethoxyisoflavone A ( 2) (32.8g), the yield was...
Embodiment 3
[0087] Synthesis of 3'-chloro-4'-amino-7-ethoxyisoflavones.
[0088] The synthesis steps are:
[0089]
[0090] ;
[0091] Dissolve 4-ethoxy-2-hydroxyacetophenone B (20g, 6.5mol) in tetrahydrofuran (100ml), add lithium hexamethyldisilazide (LiHMDS) (30ml), stir and mix thoroughly for 2h to ensure that the acyl Lithium enolate can be completely formed, then add 3-chloro-4-aminobenzoyl chloride C (22g, 6.5mol) and continue to stir for 3h to generate β-propanedione compound D; add sulfuric acid to the reaction system (30ml) and acetic acid (20ml), heat at 130°C and reflux for 4-5h, initially cool down to 24°C, adjust the pH to 8 with an alkali neutralizer, and solids precipitate; continue to cool the precipitated solids to 7°C, stir and crystallize for 1h After filtration, the filter cake was washed with ethyl acetate, recrystallized with methanol, acetone and pure water in turn, and dried in vacuo to obtain the solid compound 3′-chloro-4′-amino-7-ethoxyisoflavone A (3) (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com