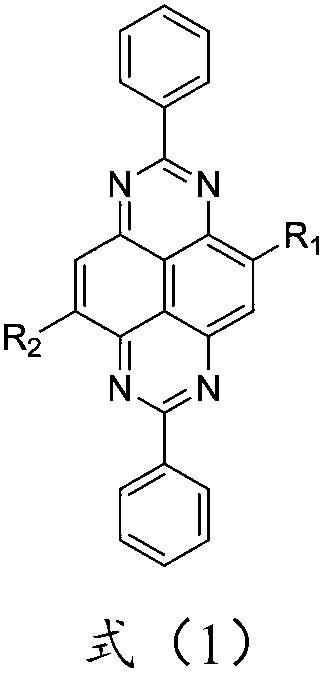
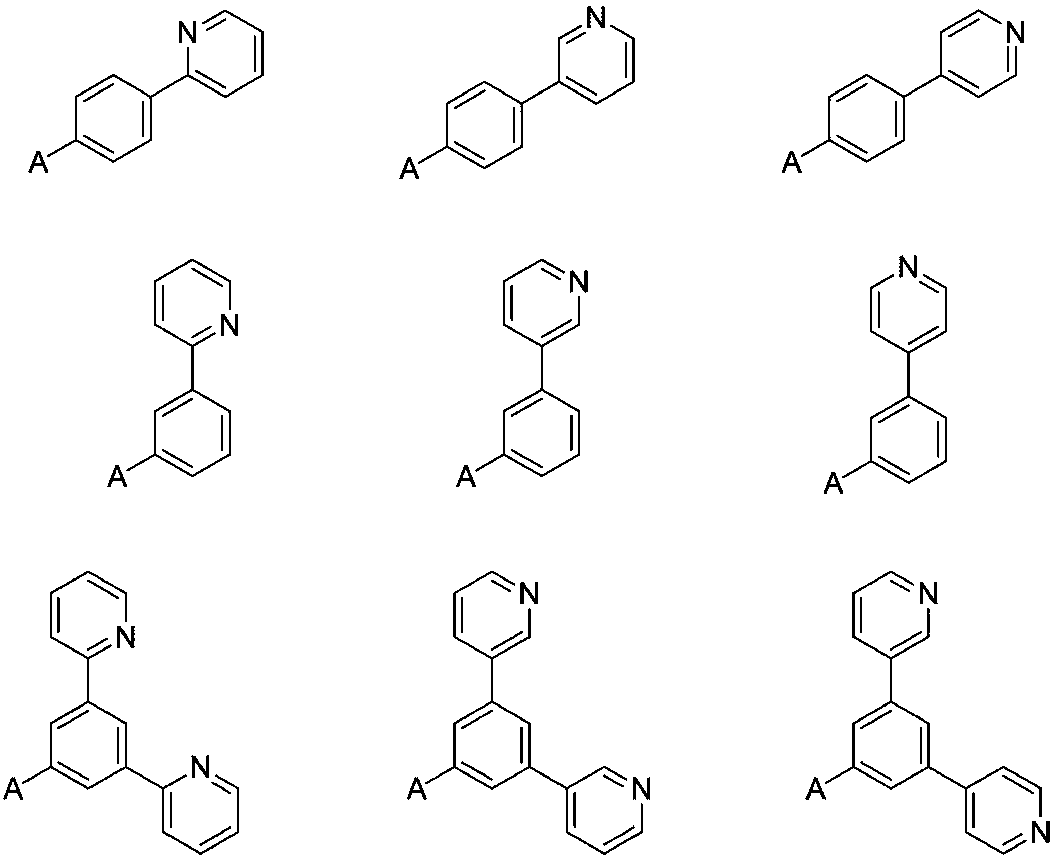
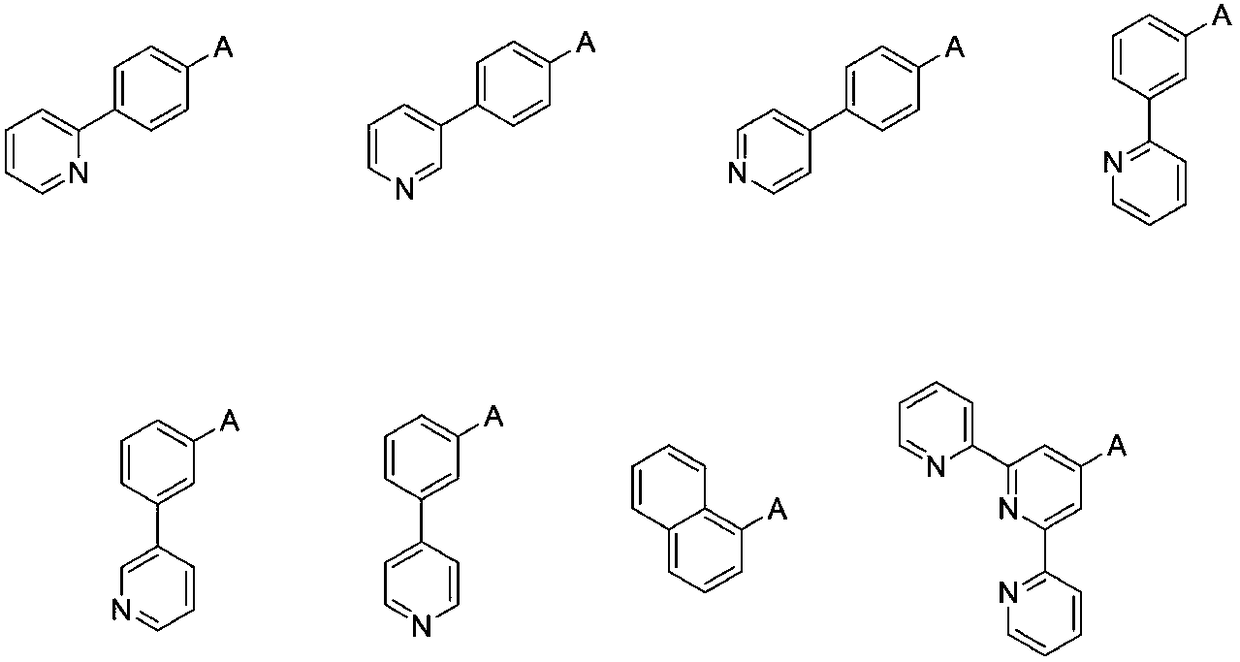
OLED electron transport material and application thereof
A technology of electron transport materials and electron transport layers, which is applied in the direction of luminescent materials, circuits, electrical components, etc., can solve the problems of slow electron transport speed, achieve the effects of excellent device performance, excellent thermal stability, and improved device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of intermediate B01, reaction equation is as follows:
[0028]
[0029] The specific operation process is: in a 1000mL three-necked flask, add compound A01 (38g, 0.20mol, CAS-RN: 187037-82-7), benzamidine (50.4g, 0.42mol), sodium ethoxide (30g, 0.44mol) , anhydrous ethanol (550g), under the protection of nitrogen, heat up to 45°C, keep the temperature for 16 hours, cool down to 25°C, pour the reaction solution into 900g deionized water, stir for 0.5h, filter with suction, rinse with 200g of absolute ethanol , collect the filter cake, use silica gel column chromatography to refine, eluent is dichloromethane:petroleum ether=2:1 (volume ratio), obtain target object B01 refined product 36.2g, yield 50.5%, high-resolution mass spectrum, positive ion Mode, formula C 24 H1 4 N 4 , the theoretical value is 358.1218, and the test value is 358.1213.
Embodiment 2
[0031] The preparation of intermediate D01, reaction equation is as follows:
[0032]
[0033] The specific operation process is: in a 500mL three-necked flask, add compound B01 (18g, 0.05mol), N,N-dimethylformamide (200g), raise the temperature to 110°C, and slowly add N-bromobutane in batches Imide (8.9g, 0.05mol) was added in about 2h, kept for 12h, cooled to 25°C, poured the reaction solution into 700g deionized water, stirred for 0.5h, filtered with suction, rinsed with 200g of absolute ethanol, collected The filter cake was purified by silica gel column chromatography, and the eluent was dichloromethane:petroleum ether=1:1 (volume ratio) to obtain 16.4 g of the target object D01 fine product, with a yield of 75%, high-resolution mass spectrometry, positive ion mode, Molecular formula C 24 h 13 BrN 4 , the theoretical value is 436.0324, and the test value is 436.0329.
Embodiment 3
[0035] The preparation of intermediate D02, reaction equation is as follows:
[0036]
[0037] The specific operation process is: in a 500mL three-necked flask, add compound B01 (18g, 0.05mol), N,N-dimethylformamide (200g), raise the temperature to 110°C, and slowly add N-bromobutane in batches Imide (17.8g, 0.1mol) was added in about 2h, kept for 16h, cooled to 25°C, poured the reaction solution into 800g deionized water, stirred for 0.5h, filtered with suction, rinsed with 200g of absolute ethanol, collected The filter cake was purified by silica gel column chromatography, and the eluent was dichloromethane:petroleum ether=1:1 (volume ratio), and further recrystallized using mesitylene as a solvent to obtain 9.6 g of the target product D02, with a yield of 37.2 %, high resolution mass spectrometry, positive ion mode, molecular formula C 24 h 12 Br 2 N 4 , theoretical value 515.9408, test value 515.9402.
[0038] Two, the synthesis embodiment of compound
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com