Application of BUIPTP (binuclear uranyl-isophthalaldehyde-tetrapyrrole) to ATP (Adenosine Triphosphate) analysis
A technology of uranyl complex and dinuclear uranyl, which is applied in the application field of dinuclear uranyl complex in ATP analysis, can solve the problems of high requirements for instruments and equipment, low precision, complicated operation and the like, and achieves good detection effect and low detection. Out-of-limit, high-precision effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
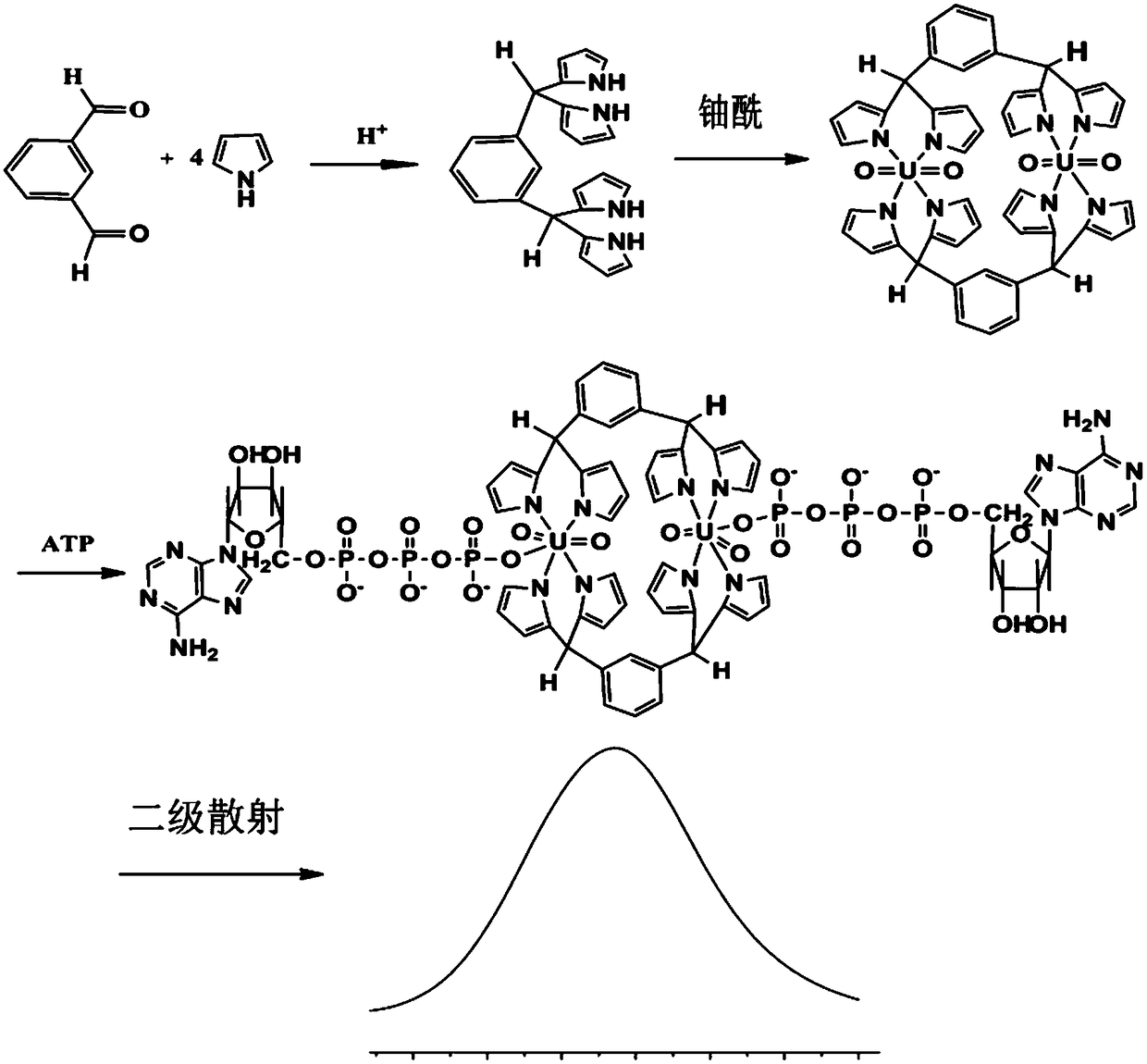
[0036] The preparation process of BUIPTP among the present invention is as follows:
[0037] (1) Add 0.12mol·L to a 250mL round bottom flask -1 100mL of hydrochloric acid, 60mmol of pyrrole and 5mmol of the corresponding aromatic aldehyde were added, and the reaction was carried out at room temperature and protected from light under magnetic stirring. The reaction system was monitored in real time by TLC. Control the appropriate reaction time, when TLC monitors that the reaction substrate aromatic aldehyde is almost completely consumed, the reaction is ended, the reaction solution is neutralized to pH=8-9 with ammonia water, filtered, and continuously washed with petroleum ether until the filtrate is colorless. Send a little of the same body obtained by suction filtration to HPLC to monitor the content of each component. The rest of the solid can be recrystallized with ethyl acetate-petroleum ether to obtain pure dipyrromethane, and then dried under reduced pressure to obtain ...
Embodiment 1
[0039] Embodiment 1: Determination of optimal conditions for detecting ATP
[0040] (1) Add 3mL of 500nmol L to a 10mL colorimetric tube -1 ATP solution.
[0041] (2) Preparation of BUIPTP detection base solution:
[0042] Accurately weigh 0.7400g of BUIPTP solid with an analytical balance, add twice-distilled water to dissolve, transfer to a 100mL volumetric flask, add water to make up to the mark, shake well to obtain 10μmol L -1 The BUIPTP detection base solution.
[0043] (3) Preparation of standard solution: 5 mL of 10 μmol L -1 The BUIPTP detection base solution was added dropwise to the solution in the above step (1).
[0044] 1.1 Determination of the maximum excitation wavelength
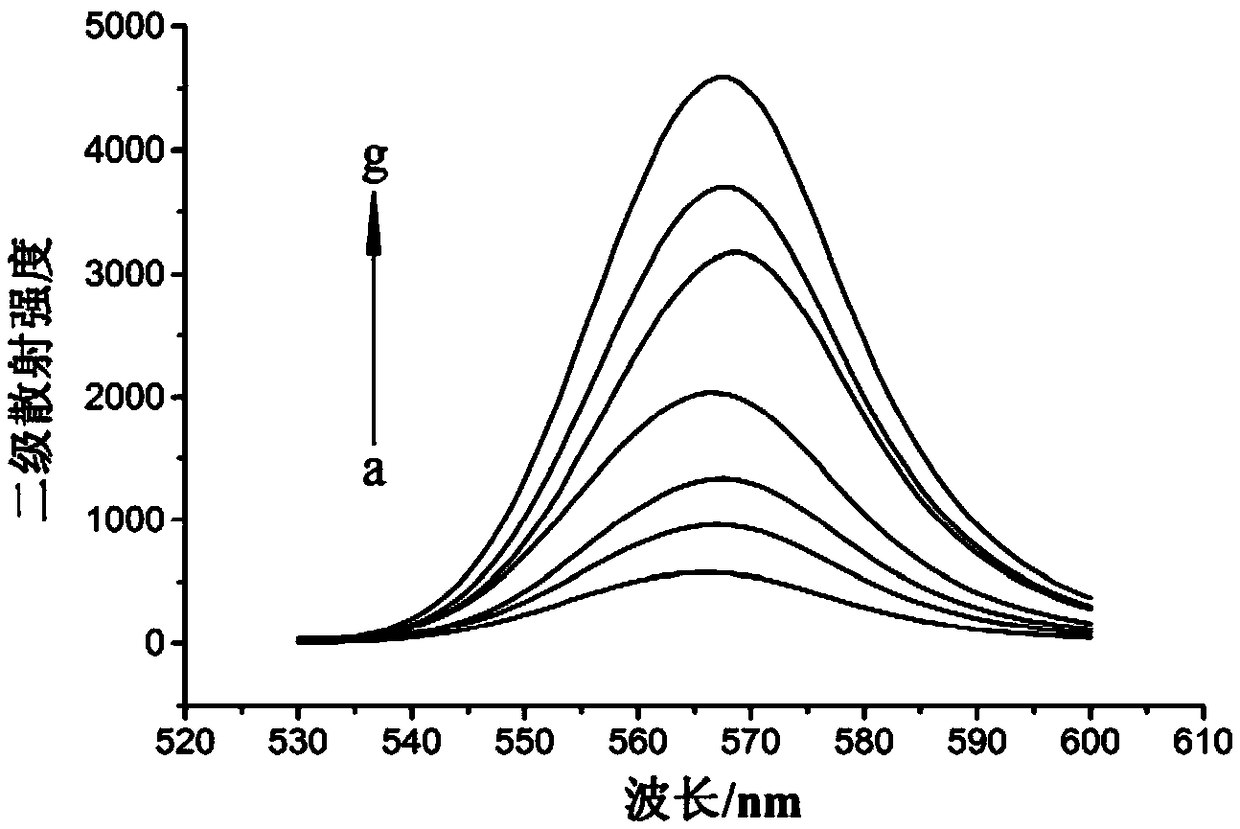
[0045] Measure the secondary scattering spectrum intensity (I SOS ), the comparison shows that the maximum excitation wavelength is 283nm, and the maximum emission wavelength is 566nm.
[0046] 1.2 Determination of optimal pH
[0047] Prepare a series of buffer solutions with a pH of...
Embodiment 2
[0050] Embodiment 2: secondary scattering experiment
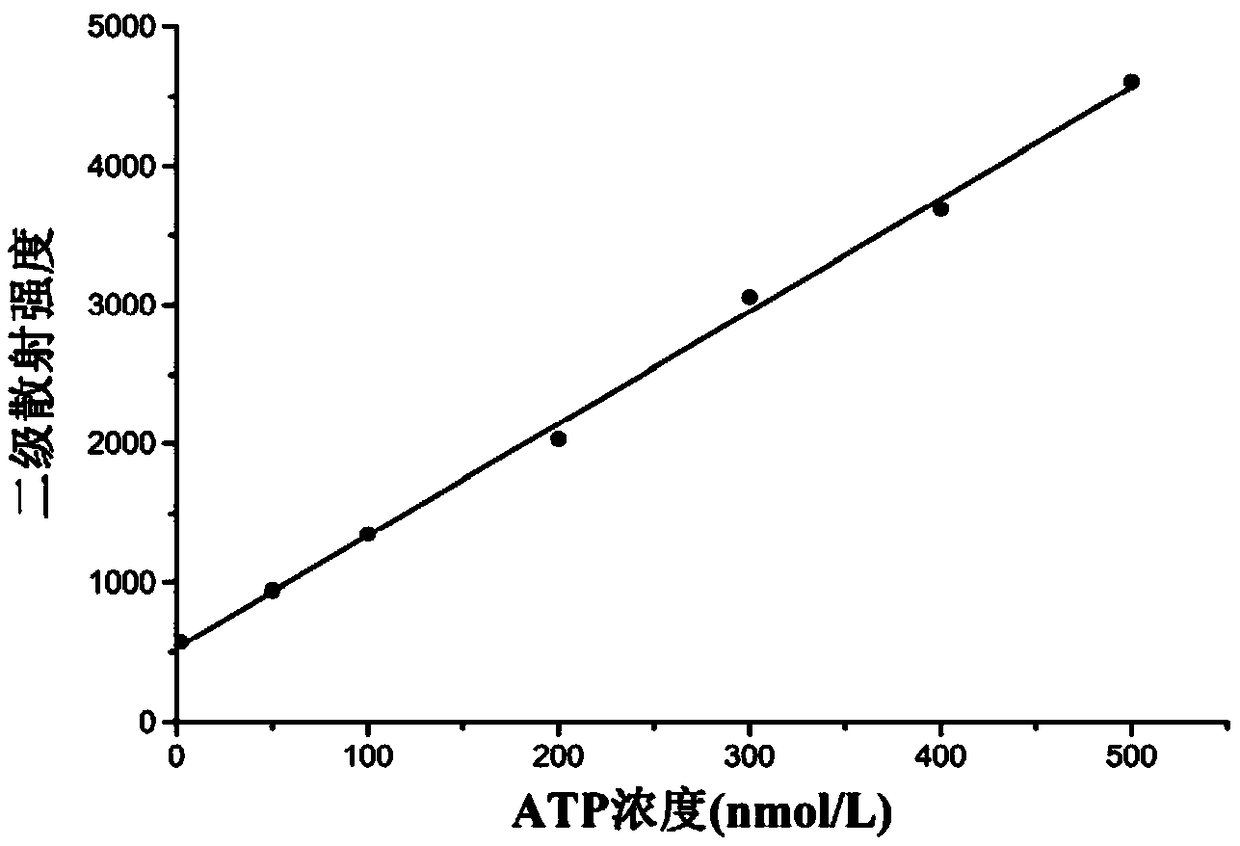
[0051] (1) Add 3mL of ATP solution and 2mL of Tris-HCl buffer (pH=7) into a 10mL colorimetric tube to obtain ATP concentrations (c) of 0, 50, 100, 200, 300, 400 and 500nmol·L -1 of the seven solutions to be tested.
[0052] (2) Preparation of BUIPTP detection base solution:
[0053] Accurately weigh 0.7400g of BUIPTP solid with an analytical balance, add twice-distilled water to dissolve, transfer to a 100mL volumetric flask, add water to make up to the mark, shake well to obtain 10μmol L -1 The BUIPTP detection base solution.
[0054] (3) Preparation of standard solution: 5 mL of 10 μmol L -1 The BUIPTP detection base solution was added dropwise to each solution to be tested to obtain the corresponding seven standard solutions. The total dropping time was 10 minutes and incubated for 30 minutes.
[0055] Among them, we have learned through experiments that the secondary scattering signal is stronger when BUIPTP is add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com