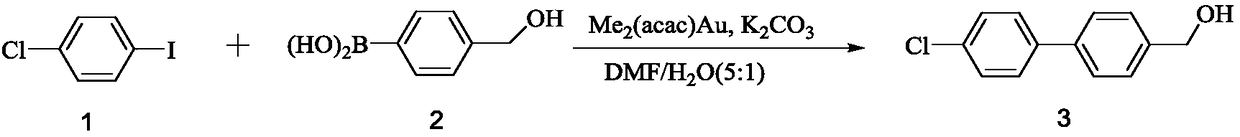Preparation method and application of dimethyl acetylacetone gold
A technology of acetylacetone and dimethyl, which is applied in the field of complex catalysis and achieves the effects of easy operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] (1) Dissolve gold chloride (1 mmol) in ether (10 mL), add methyl iodide (2.5 mmol), and stir at room temperature for 2 hours to obtain reaction solution A;
[0015] (2) Add potassium tert-butoxide (3mmol) to acetylacetone (3mmol) and stir at room temperature for 10min to obtain reaction solution B;
[0016] (3) Under an ice bath, mix the reaction solution A and the reaction solution B, naturally return to room temperature, stir and react for 30 minutes, dilute with ether, wash with water, and concentrate the organic layer (ether layer) under reduced pressure and vacuum dry to obtain two Methyl (acetylacetonate) gold (III) (294 mg, yield is about 90.1%, melting point 82-84°C).
Embodiment 2
[0018] (1) Dissolve gold chloride (1 mmol) in ether (15 mL), add methyl iodide (3.0 mmol), and stir at room temperature for 1 hour to obtain reaction solution A;
[0019] (2) Add potassium tert-butoxide (5 mmol) to acetylacetone (5 mmol), and stir at room temperature for 15 minutes to obtain reaction solution B;
[0020] (3) Under ice bath, mix reaction solution A and reaction solution B, return to room temperature naturally, stir and react for 40 minutes, dilute with ether, wash with water, and concentrate the organic layer (ether layer) under reduced pressure and dry in vacuo to obtain two Methyl (acetylacetone) gold (III) (301mg, yield is about 92.3%, melting point 82-84°C).
Embodiment 3
[0022]
[0023] Take compound 2 (1.2mmol) and dissolve it in DMF / H 2 O mixed solvent (DMF: H 2 O=5:1, v / v), at room temperature, add dimethyl(acetylacetone) gold (Ⅲ) (0.1mmol) and stir for 5min, add compound 1 (1.0mmol) and potassium carbonate (5mmol), continue to room temperature After stirring and reacting for 15 minutes, the reaction solution was poured into ice water to separate out solids, filtered, the filter cake was washed with water and n-hexane in turn, and dried under vacuum to obtain compound 3 (205 mg, yield is about 93.7%) 1 H NMR(400MHz, CDCl 3 ):δ7.56-7.43(m,8H,Ph- H ),4.75(s,2H,C H 2 ),1.80(s,1H,O H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com