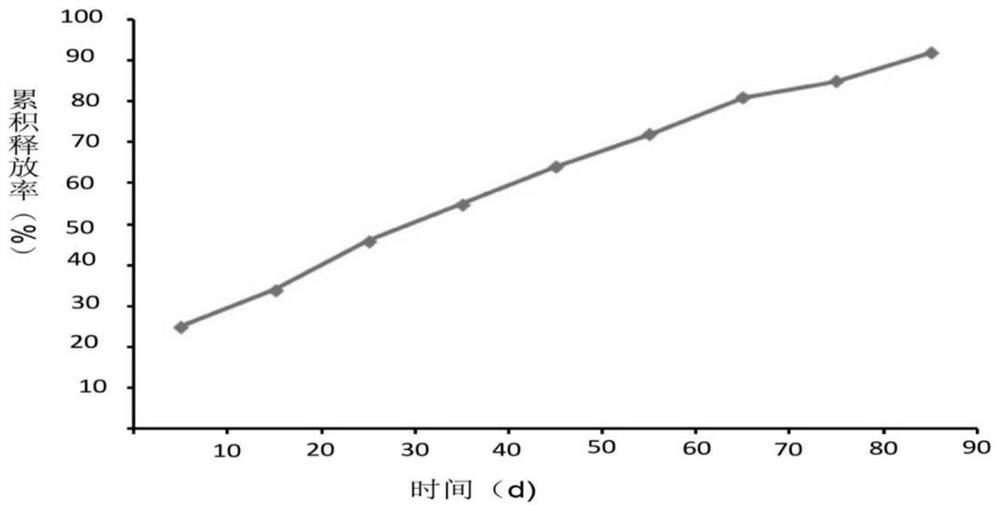
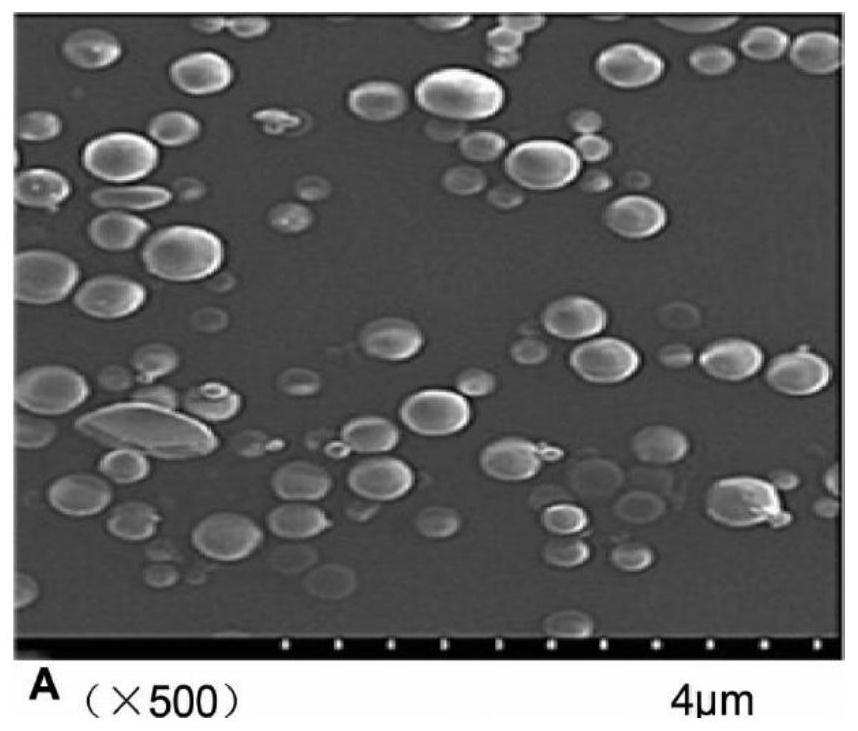
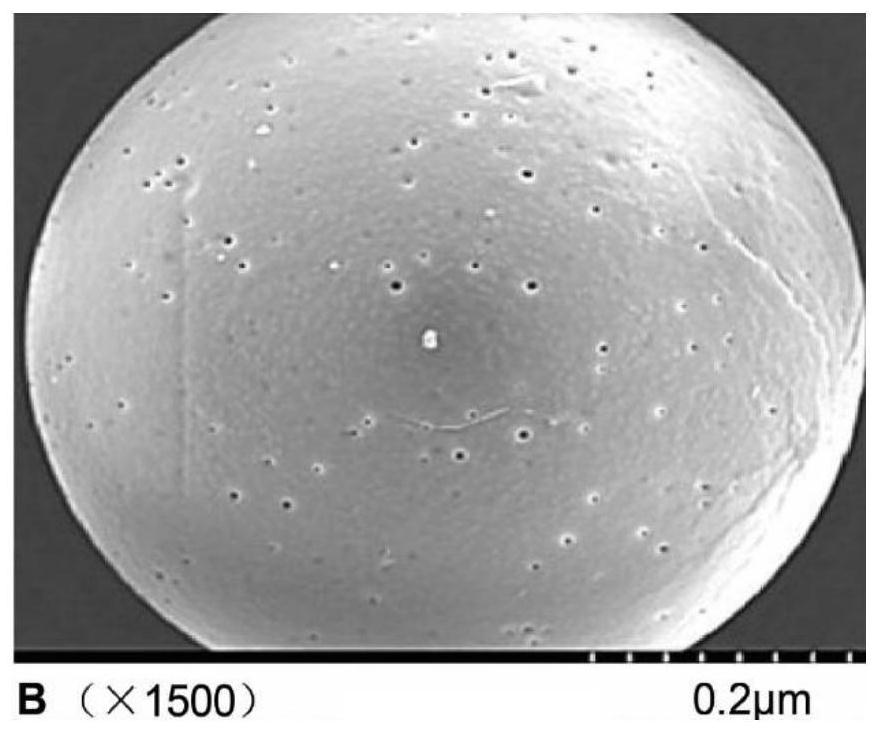
Anti-NGF antibody slow-release microspheres for reducing airway hyperreactivity and preparation method thereof
A technology of airway hyperresponsiveness and slow-release microspheres, which is applied in the direction of antibodies, antibody medical ingredients, and medical preparations of non-active ingredients, etc., can solve the problem of no anti-NGF antibody drugs, etc., to reduce airway hyperresponsiveness , reduce airway inflammation, does not affect the effect of systemic immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Anti-NGF antibody micronization
[0035] The concentration of the bovine serum albumin solution configured is 0.5mg / mL, the concentration of the anti-NGF antibody solution is 2mL / kg, 300 μg of the anti-NGF antibody solution is mixed with 0.5 ml of bovine serum albumin solution, and then lyophilized, and then freeze-dried Conditions are freezing at -20°C for 3 hours; drying at 20°C for 12 hours. After the mixture was lyophilized, it was uniformly dispersed in the bovine serum albumin matrix to form anti-NGF antibody powder.
[0036] 2. Anti-NGF microencapsulation
[0037] S1: Mix polylactic acid and glycolic acid in a weight ratio of 1:2 to prepare polylactic acid;
[0038] S2: Mix 10 mg of NGF antibody micropowder with 300 mg of polylactic acid, dissolve in 1 mL of dichloromethane to form an oil phase;
[0039] S3: Prepare 8 mL of 2% polyvinyl alcohol solution, add it to the oil phase, and stir at high speed for 20 seconds to form an S / O / W type double emulsion;
...
Embodiment 2
[0043] 1. Anti-NGF antibody micronization
[0044]The concentration of the bovine serum albumin solution configured is 0.6mg / mL, the concentration of the anti-NGF antibody solution is 4mL / kg, 300 μg of the anti-NGF antibody solution is mixed with 0.5 ml of bovine serum albumin solution, and then lyophilized, and then freeze-dried Conditions were freezing at -30°C for 3 hours; drying at 20°C for 12 hours. After the mixture was lyophilized, it was uniformly dispersed in the bovine serum albumin matrix to form anti-NGF antibody powder.
[0045] 2. Anti-NGF microencapsulation
[0046] S1: Mix polylactic acid and glycolic acid in a weight ratio of 1:5 to prepare polylactic acid;
[0047] S2: Mix 20mg of NGF antibody micropowder with 300mg of polylactic acid, dissolve in 3mL of dichloromethane to form an oil phase;
[0048] S3: Prepare 15 mL of 2% polyvinyl alcohol solution, add it to the oil phase, and stir at high speed for 60 seconds to form an S / O / W type double emulsion;
[...
Embodiment 3
[0051] 1. Anti-NGF antibody micronization
[0052] The concentration of the bovine serum albumin solution configured is 0.8mg / mL, the concentration of the anti-NGF antibody solution is 6mL / kg, 300 μg of the anti-NGF antibody solution is mixed with 0.5 ml of bovine serum albumin solution, and then lyophilized. Conditions are freezing at -20°C for 3 hours; drying at 20°C for 12 hours. After the mixture was lyophilized, it was uniformly dispersed in the bovine serum albumin matrix to form anti-NGF antibody powder.
[0053] 2. Anti-NGF microencapsulation
[0054] S1: Mix polylactic acid and glycolic acid in a weight ratio of 1:3 to prepare polylactic acid;
[0055] S2: Mix 12mg of NGF antibody micropowder with 300mg of polylactic acid, dissolve in 2mL of dichloromethane to form an oil phase;
[0056] S3: Prepare 10 mL of 2% polyvinyl alcohol solution, add it to the oil phase, and stir at high speed for 30 seconds to form an S / O / W type double emulsion;
[0057] S4: Prepare 10% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com