Blood plasma gene detection kit for early diagnosis of laryngeal squamous cell carcinoma
A technique for early diagnosis of squamous cell carcinoma, applied in the determination/examination of microorganisms, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
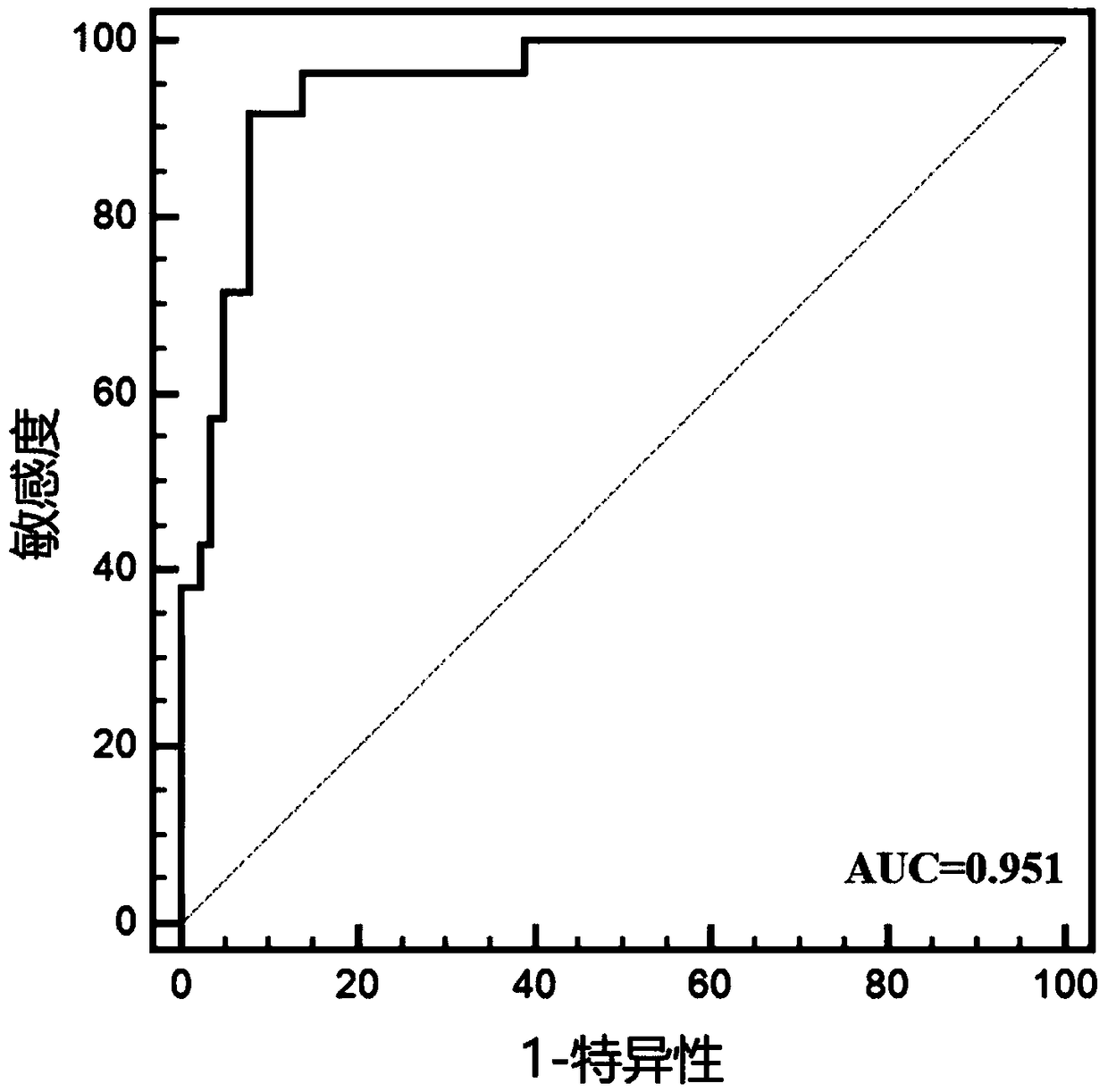
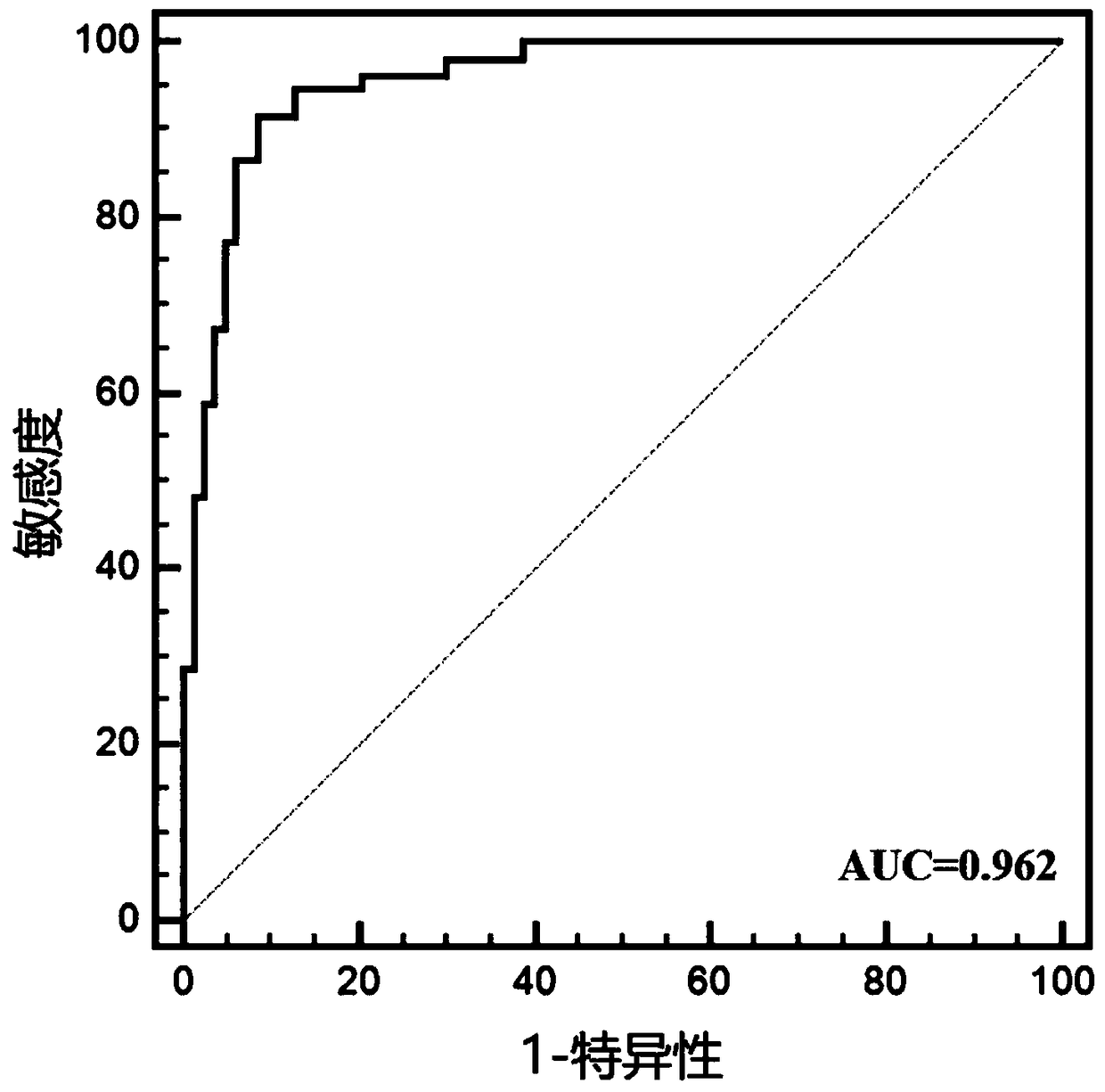
[0025] Example 1: Preliminary verification of the diagnostic efficacy of miRNA markers for LSCC
[0026] 1. Subjects
[0027] This study included 75 newly diagnosed LSCC patients diagnosed by WHO histopathology in Jiangsu Provincial People's Hospital from June 2015 to June 2017. LSCC patients with other tumors or acute infections were excluded. Among the patients, there were 45 males and 30 females. At the same time, 75 age- and gender-matched healthy people who underwent physical examination at the Health Examination Center of Jiangsu Provincial People's Hospital were included as controls, including 45 males and 30 females, and there was no statistical difference in age from LSCC patients.
[0028] 2. Experimental method
[0029] 1. Plasma collection
[0030] Collect 2ml of venous blood and place it in an anticoagulant tube treated with dipotassium ethylenediaminetetraacetic acid. After anticoagulant treatment, divide it into a 2ml enzyme-free centrifuge tube, centrifuge ...
Embodiment 2
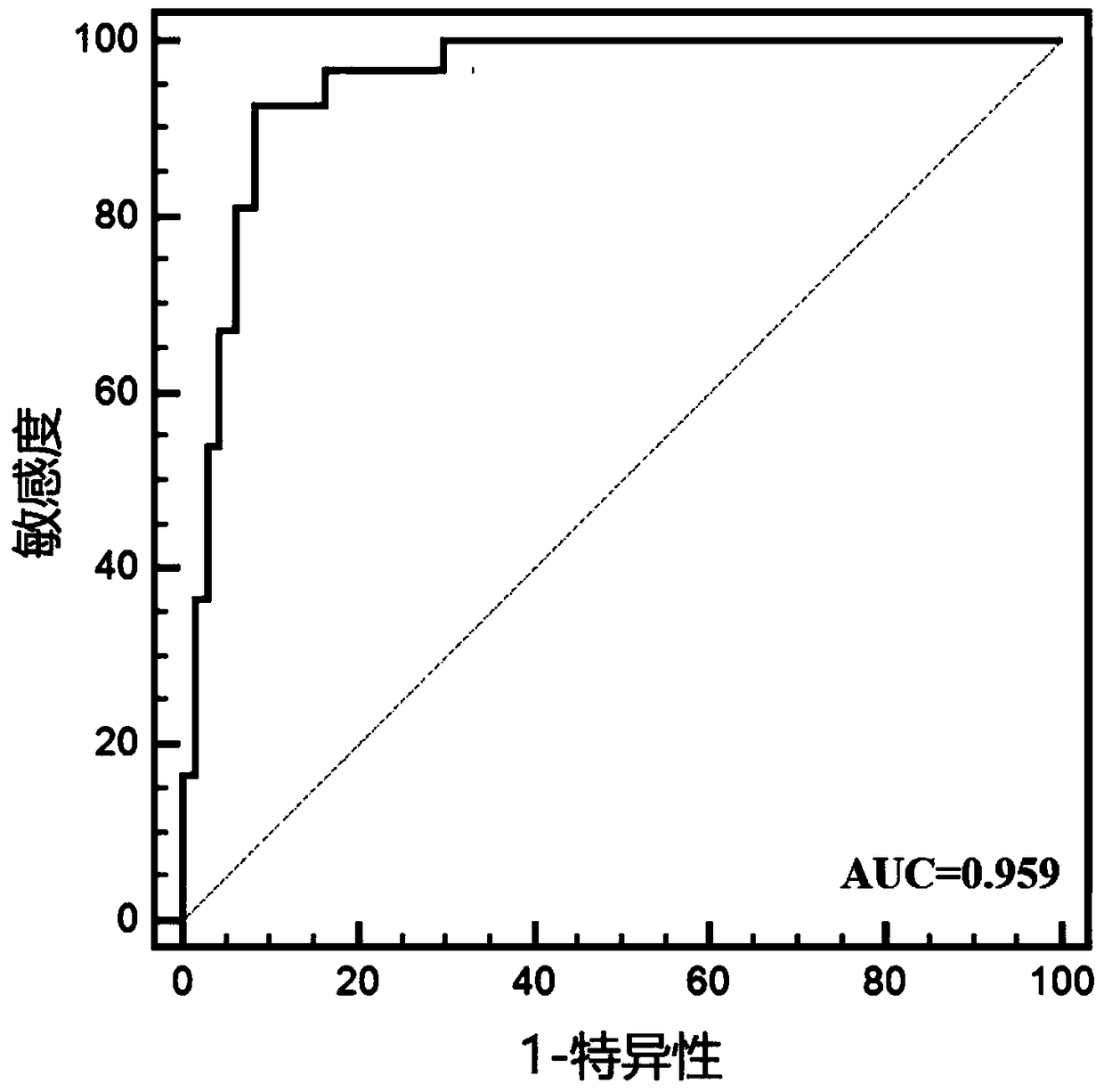
[0057] Example 2: Further multi-center validation of miRNA markers for LSCC diagnostic efficacy
[0058] 1. Subjects
[0059] This study included 48 newly diagnosed LSCC patients diagnosed by WHO histopathology in the Affiliated Hospital of Southwest Medical University from June 2015 to June 2017. LSCC patients with other tumors or acute infections were excluded. Among the patients, there were 30 males and 18 females. At the same time, 48 age- and sex-matched healthy people who underwent physical examination at the Health Examination Center of the Affiliated Hospital of Southwest Medical University were included as controls, including 30 males and 18 females, and there was no statistical difference in age from LSCC patients.
[0060] 2. Experimental methods and results
[0061] 1. Plasma collection
[0062] Collect 2ml of venous blood and place it in an anticoagulant tube treated with dipotassium ethylenediaminetetraacetic acid. After anticoagulant treatment, divide it int...
Embodiment 3
[0078] Embodiment 3: LSCC diagnostic kit
[0079] The LSCC diagnostic kit can contain the detection of hsa-miR-544a, hsa-miR-4726 and hsa-miR-135a-5p, or the detection of hsa-miR-544a, hsa-miR-4726 and hsa-miR-2278, or the detection of Primers for hsa-miR-544a, hsa-miR-636 and hsa-miR-597-5p also contain internal references and enzymes and reagents required for RT-PCR.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com