Fluorescent dye having phenazine condensed structure and synthesis method of same
A fluorescent dye and fusion technology, applied in the field of fluorescent dyes, can solve the problems of small Stokes shift value and complex synthesis method, and achieve the effects of reducing the interference of self-quenching, simple synthesis steps and high reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
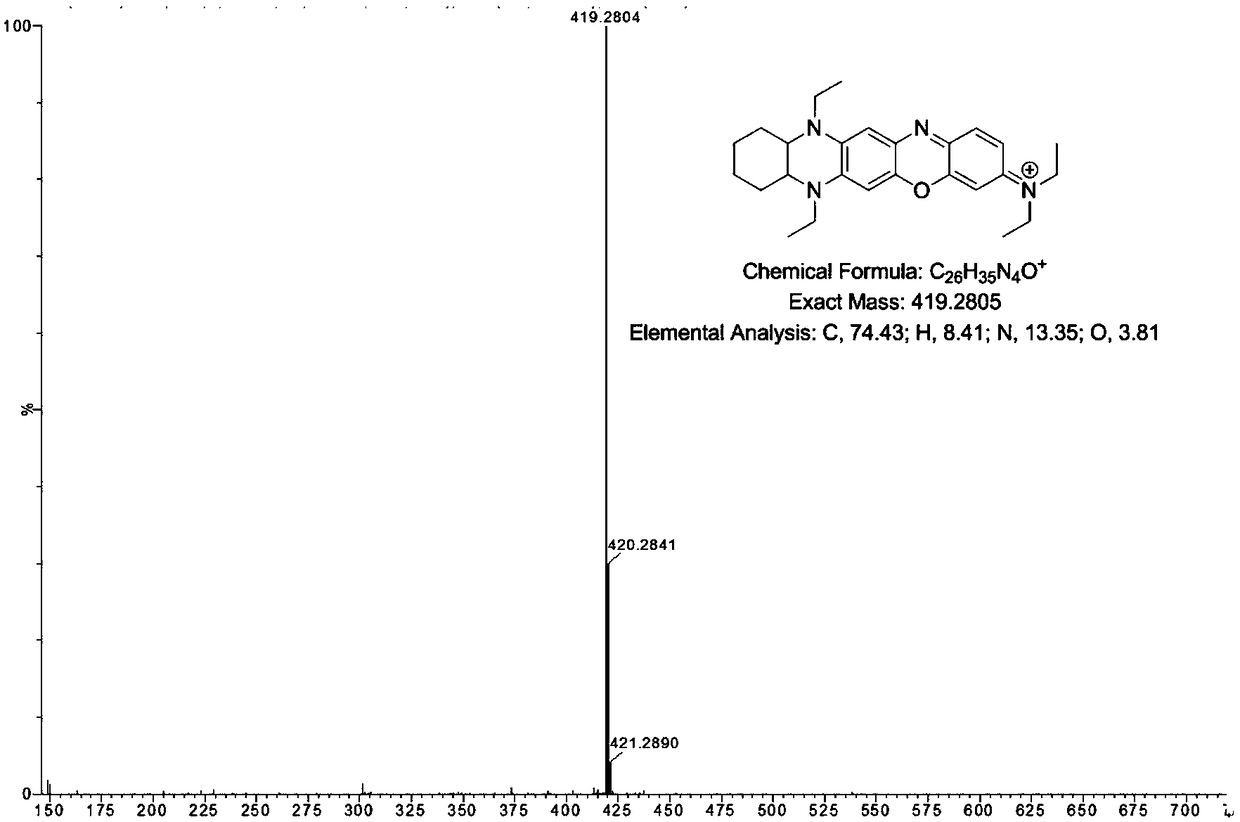
[0048] Example 1 Synthesis of fluorescent dye (DQF-593) with fused structure of type I phenazine
[0049] Dissolve 12 mmol of 4-methoxy-o-phenylenediamine in 30 mL of ethanol, add 12 mmol of cyclohexanedione and react at 60°C for 8 hours. After the reaction is completed, concentrate and pass through a neutral alumina column to obtain 7-methoxy-1, 2,3,4-Tetrahydrophenazine.
[0050] Dissolve 5mmol of 7-methoxy-1,2,3,4-tetrahydrophenazine in 150mmol of anhydrous toluene, slowly add 50mmol of sodium borohydride and 150mmol of acetic acid at 0°C, and then react at 0°C for 1h , after the reaction is completed, warm up to room temperature first, and then heat at 110°C to reflux for 6 hours; after the reaction, slowly pour into water and extract with ethyl acetate, the organic phase is washed, dried, and concentrated to obtain a yellow viscous Intermediate (5,10-diethyl-7-methoxy-1,2,3,4,4a,5,10,10a- octahydrophenazine) (its structure is as shown in (2) in the reaction equation Sho...
Embodiment 2
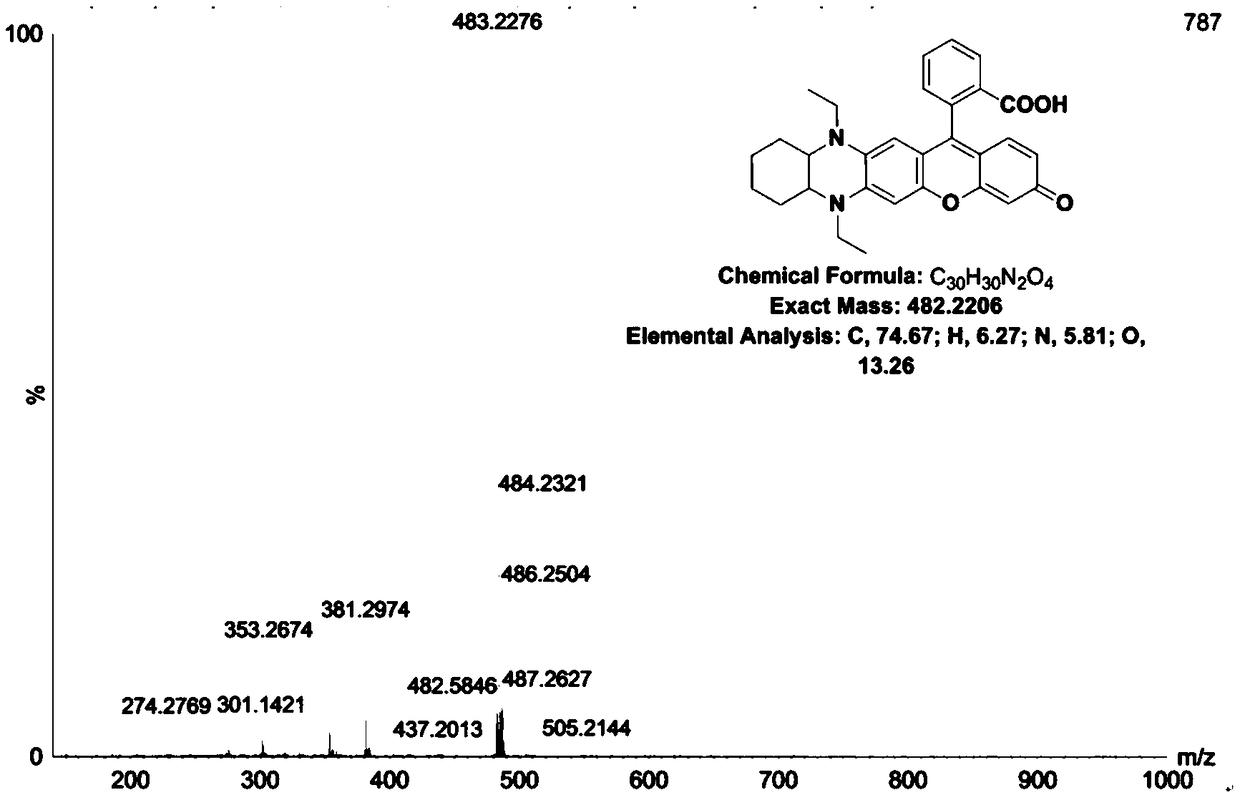
[0053] Example 2 Synthesis of fluorescent dye (DQF-562) with fused structure of type II phenazine
[0054]
[0055] With 2-carboxy-2', 4'-dihydroxybenzophenone (its structure is as shown in (1) in the reaction equation) 0.2mmol and 5,10-diethyl-7- Dissolve 0.2mmol of methoxy-1,2,3,4,4a,5,10,10a-octahydrophenazine in 5mL of methanesulfonic acid, heat to 90°C for 6h, after the reaction, pour the reaction liquid Put into ice water, then add 6mL of perchloric acid and let it stand until the solid precipitation is complete, then wash the solid, filter with suction, and dry to obtain the crude product. After the crude product is separated by column, the brown product type II phenazine condensed Structure of the fluorescent dye (DQF-562), with high resolution mass spectrometry ( figure 2 ) and NMR analysis. 1 H NMR (400MHz, MeOD) δ8.31(d, J=6.0Hz, 1H), 7.80(2H), 7.38(d, J=7.6Hz, 1H), 7.18(d, J=8.1Hz, 1H), 7.12(s,1H),7.05(s,1H),6.93(d,J=7.5Hz,1H),6.09(d,J=7.6Hz,1H),3.89(s,1H),3...
Embodiment 3
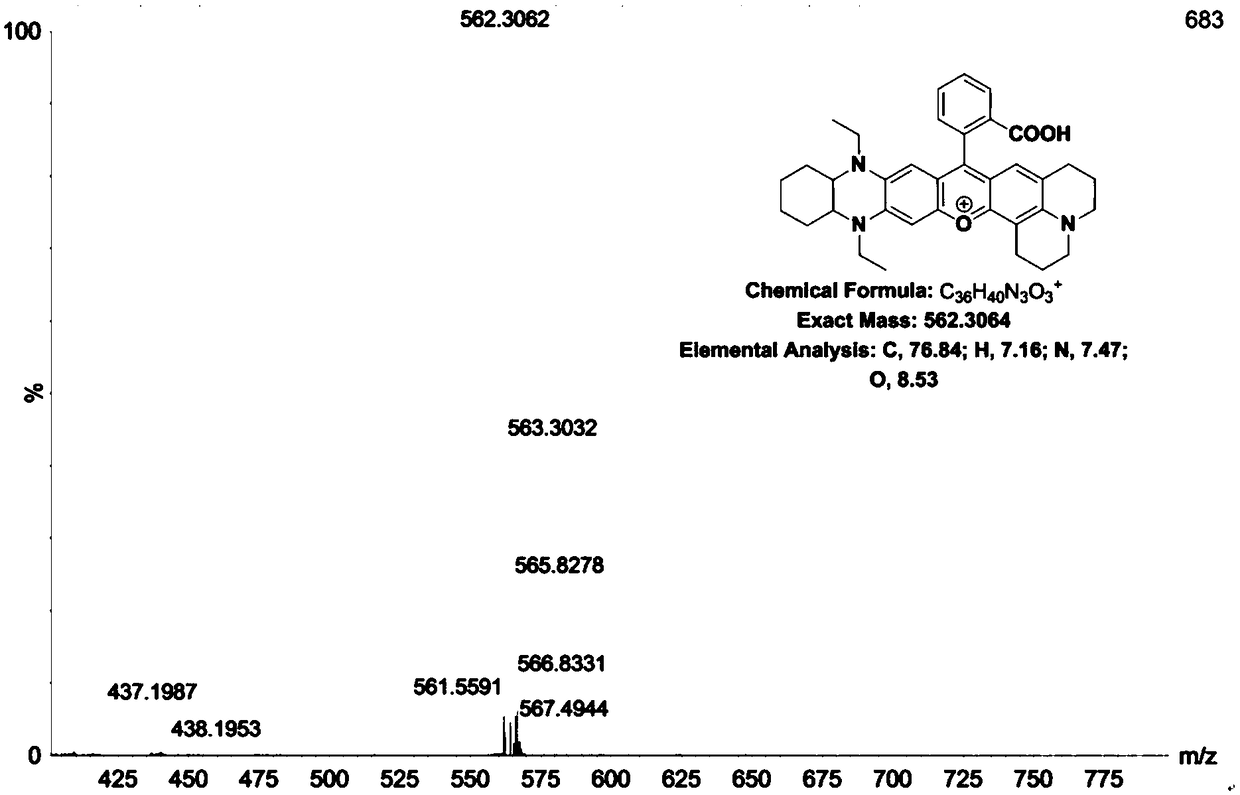
[0056] Example 3 Synthesis of fluorescent dye (DQF-683) with fused structure of type III phenazine
[0057] Dissolve 12mmol of o-phenylenediamine in 30mL of ethanol, add 14mmol of cyclohexanedione, then react at 60°C for 8h, the mixture is concentrated and passed through a neutral alumina column to obtain 1,2,3,4-tetrahydrophenazine.
[0058] Dissolve 5 mmol of 1,2,3,4-tetrahydrophenazine in 30 mL of anhydrous toluene, slowly add 50 mmol of sodium borohydride and 30 mL of acetic acid at 0°C, and then react at 0°C for 1 hour. After the reaction is completed, first The temperature was raised to room temperature, and then heated to reflux at 110°C for 7 hours; after the reaction, it was slowly poured into water, extracted with ethyl acetate, and the organic phase was washed, dried, and concentrated to obtain a yellow viscous intermediate 5,10- Diethyl-1,2,3,4,4a,5,10,10a-octahydrophenazine (its structure is shown in (2) in the reaction equation).
[0059]
[0060] 4-diethylam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com