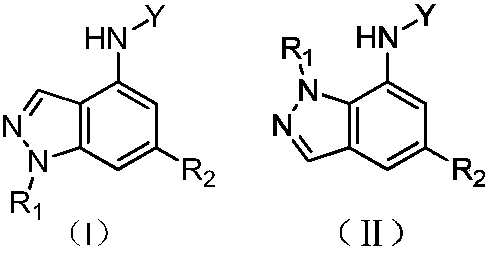
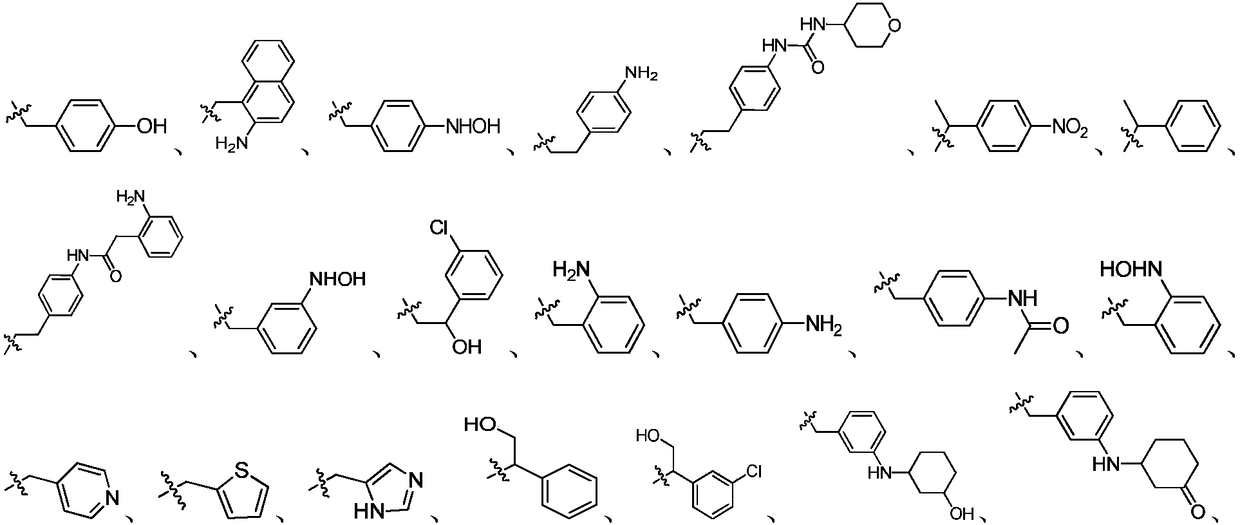
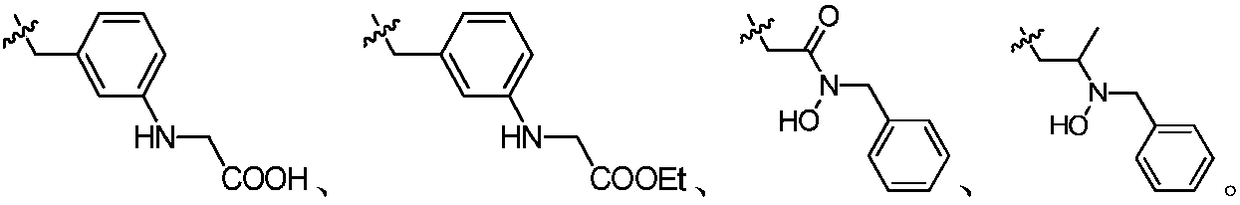
Indazole compounds and application thereof to preparation of IDO inhibitors
A technology of compounds and mixtures, applied in the fields of drug combination, organic chemistry, antiviral agents, etc., can solve the problems of reducing tryptophan concentration, inhibiting killing effect, stagnation of synthesis, etc., and achieving excellent inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091]The synthesis of embodiment 1 compound LWQ-137, LWQ-139, LWQ-140
[0092] The synthetic route is as follows:
[0093]
[0094] 1 Synthesis of Compound LWQ-139
[0095] Dissolve starting material 13b (50mg, 0.2994mmol), starting material 14 (50mg, 0.3892mmol) and dihydropyridine ester (110mg, 0.4192mmol) in DCM:MeOH=6:2ml, add trifluoroacetic acid (23μL, 0.2994 mmol). After addition, open to 40°C and stir at reflux for 3h. TLC showed that the reaction of the raw materials was complete, and finally 53 mg of a beige solid was obtained, with a yield of 65.3%.
[0096] 1 H NMR(400MHz,DMSO)δ12.82(s,1H),9.31(s,1H),8.22(s,1H),7.20(s,1H),6.73(d,J=8.4Hz,2H),6.67 (s,1H),5.95(s,1H),4.31(d,J=5.8Hz,2H),3.38(s,1H),2.54–2.47(m,1H). 13 C NMR (400MHz, DMSO) δ156.69, 143.40, 141.68, 133.11, 132.52, 129.79, 128.69, 115.53, 112.35, 98.61, 96.97, 46.23, 30.96. HRMS (AP-ESI) Calcd.for C 14 h 12 ClN 3 O:296.0567(M+Na) + .Found: 296.0025.
[0097] 2 Synthesis of compounds LWQ-137 a...
Embodiment 2
[0102] The synthesis of embodiment 2 compound LWQ141, LWQ-173, LWQ-175, LWQ-176, LWQ-177, LWQ-183
[0103] The synthetic route is as follows:
[0104]
[0105] Starting material 7 (212.0 mg, 1.0 mmol) and starting material m-nitrobenzaldehyde (181.2 mg, 1.2 mmol) were dissolved in DCM (10 mL) and methanol (5 mL), dihydropyridinate (354.2 mg, 1.4 mmol) was added, Trifluoroacetic acid (74.5 μL, 1.0 mmol) was added dropwise, and the reaction was refluxed at 40° C. for 6 hours. TLC detects that after the reaction of the raw materials is complete, the reaction is cooled to room temperature, and solid NaHCO is added. 3 Adjust the pH to about 7-8, add silica gel to the reaction solution, spin dry, and pass through the column (DCM:MeOH=100:1-80:1-60:1) to obtain 266.4 mg of yellow solid compound 15c with a yield of 81.5%.
[0106] Other compounds were synthesized using compound 7 and corresponding aldehyde raw materials, and were obtained by referring to the synthesis method of c...
Embodiment 3
[0114] The synthesis of embodiment 3 compound LWQ-170, LWQ-171, LWQ-172
[0115] The synthetic route is as follows:
[0116]
[0117]Synthesis of compound LWQ-171: Dissolve compound 15c (208.2mg, 0.60mmol) in ethanol (3mL) and water (3mL), add ammonium chloride (16.5mg, 0.3mmol), add iron powder (166.7mg , 2.98mmol), reacted at 80°C for 30 minutes, filtered the reaction solution while it was hot, spin-dried the solvent under reduced pressure, and passed the column (DCM:MeOH=30:1) to obtain 166.0mg of a light yellow solid with a yield of 87.3%.
[0118] Compounds LWQ-170 and LWQ-172 were prepared using compounds 15b and 15d as raw materials, respectively, according to the synthetic method of compound LWQ-171.
[0119] LWQ-170: yellow solid 22.7mg, yield 25.7%. 1 H NMR (400MHz, DMSO) δ12.90(s, 1H), 8.11(s, 1H), 6.88(d, J=8.4Hz, 2H), 6.43(d, J=8.4Hz, 2H), 6.36(s ,1H),5.89(s,2H),4.81(s,2H),4.01(s,2H). 13 C NMR(101MHz,DMSO)δ146.67,143.43,142.13,132.68,128.04,127.96,122.06,12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com