Selenium-containing compound and use thereof
A technology for compounds and uses, applied in the field of preparing cancer chemopreventive agents and therapeutic agents, which can solve the problems of further improvement of anticancer efficacy, limited structure types of compounds, limited anticancer spectrum, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The preparation method of the invention is simple, the yield is high, and N-(ethylselenocyanate)-N'-(methylsulfinylbutyl)urea can be easily prepared.
[0023] In another aspect of the present invention, there is provided a pharmaceutical composition comprising a compound of formula I of the present invention or a pharmaceutically acceptable salt thereof, and optionally a pharmaceutically acceptable excipient and / or carrier. In the pharmaceutical composition of the present invention, in addition to the compound of formula I of the present invention or a pharmaceutically acceptable salt thereof, other pharmaceutically active ingredients may also be included. The pharmaceutical compositions of the present invention may be prepared by conventional techniques, such as those described in Remington: The Science and Practice of Pharmacy, 19th Edition, 1995, which is incorporated herein by reference. The compositions may be presented in conventional forms, such as capsules, tabl...
Embodiment 1
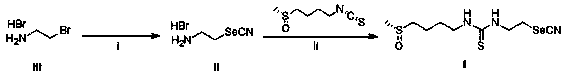
[0057] Embodiment 1: Synthesis of 1-selenocyanoethylamine hydrobromide (compound of formula II)
[0058] Add 2-bromoethylamine hydrobromide (1.74g, 8.5 mmol) and 20ml of anhydrous acetonitrile into a three-necked flask, add potassium selenocyanate (1.24g, 8.6 mmol) under nitrogen protection at room temperature, and stir for 24 hours. Remove the solvent by distillation under reduced pressure, add 30ml of dichloromethane to continue the reaction at room temperature for 15-20 minutes, and distill under reduced pressure again to obtain the crude product, column chromatography (mobile phase: ethyl acetate: petroleum ether = 10:1 (V:V)) 1.65 g of a yellow powdery solid (compound II) was obtained, with a yield of 84%.
[0059] nuclear magnetic resonance 1 H NMR (400 MHz, CDCl 3 ) δ: 4.03 (br s, 2H), 3.67 (s, 2H).
[0060] MS [ESI]: Calculated (C 3 h 7 BrN 2 Se) + , 150; found value: 151.
Embodiment 2
[0061] Embodiment 2: the synthesis of N-(ethyl selenocyanine)-N'-(methylsulfinyl butyl)urea (formula I compound)
[0062] Dissolve 1-selenocyanoethylamine hydrobromide (428 mg, 1.86 mmol) in anhydrous dichloromethane, add triethylamine (513 mg, 5.58 mmol), and add sulforaphane (300 g, 1.86 mmol), heated to 50°C and continued to react for 2 hours. The reaction was complete as detected by TCL, the solvent was distilled off under reduced pressure, and purified by column chromatography (mobile phase: ethyl acetate: dichloromethane = 2:1 (V:V)) to obtain 0.54 g of a white powdery solid (compound of formula I), Yield 89%.
[0063] nuclear magnetic resonance 1H NMR (400 MHz, CD 3 OD) δ: 3.88~3.84 (t, 2H), 3.67~3.65 (t,2H), 3.48~3.44 (t, 2H), 2.91~2.83(m, 2H), 2.65 (s, 3H), 1.91~1.87 (m, 4H); 13 CNMR (75 MHz, CD 3 OD) δ: 180.4, 160.5, 57.7, 54.1, 45.2, 38.6, 27.3, 20.6, 20.2.
[0064] MS [ESI]: Calculated value (C 9 h 17 N 3 OS 2 Se) + , 327; Found: 328.
[0065] in vitro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Nuclear magnetic resonance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com