Preparation method for rocuronium bromide injection in low impurity level
A technology of rocuronium bromide and injection, which is applied in the field of medicine and can solve the problems of increasing impurity content and instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] After research it was found that:
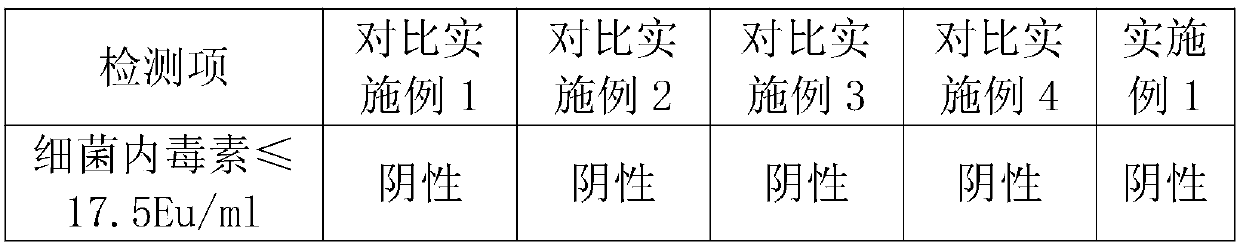
[0023] 1. When the bacterial endotoxin in the raw and auxiliary materials is strictly controlled, the preparation process does not need to add activated carbon for adsorption and heat removal, and the endotoxin of the final product also meets the standard requirements.
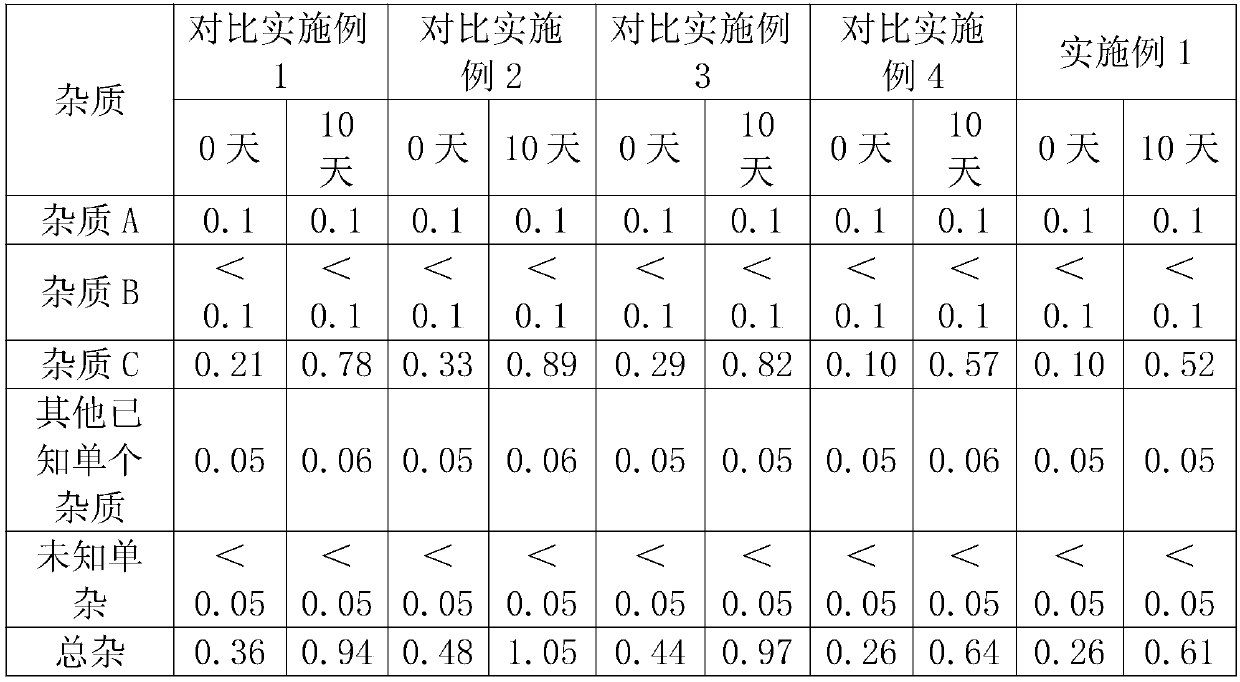
[0024] 2. The main degradation impurity of rocuronium bromide is impurity C. Rocuronium bromide is unstable under high temperature conditions. If it is treated at 121°C for 20 minutes, the impurity content of impurity C will increase by 3 times.
[0025] 3. Rocuronium bromide is stable under acidic conditions. After being treated with 1mol / L hydrochloric acid, the content of impurity C does not change significantly, but it is unstable under alkaline conditions, and the content of impurity C increases significantly. Therefore, in the preparation process, reducing the pH of the solution is beneficial to ensure the stability of rocuronium bromide injection.
[0026] 4. ...
Embodiment 1
[0053] The composition and consumption of the injection in the table 5 embodiment 1
[0054] Element
Dosage
10.0
Sodium acetate
2.0
glacial acetic acid
Appropriate amount
3.3
Appropriate amount
Water for Injection
Appropriate amount
Total
1000.0
[0055] According to the above table 5, the preparation method is as follows: measure about 850 g of water for injection cooled to below 25°C, aerate with nitrogen, cool the solution to 8-12°C, adjust the pH to 2.9-3.1 with glacial acetic acid to obtain solution A, and Add the prescribed amount of rocuronium bromide to solution A, stir and dissolve to obtain solution B, add the prescribed amount of sodium acetate and sodium chloride to solution B, adjust the pH of the solution to 2.9-3.1 with glacial acetic acid, and add water for injection to 1000.0g , adjust the pH of solution B to 3.9-4.1 wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com