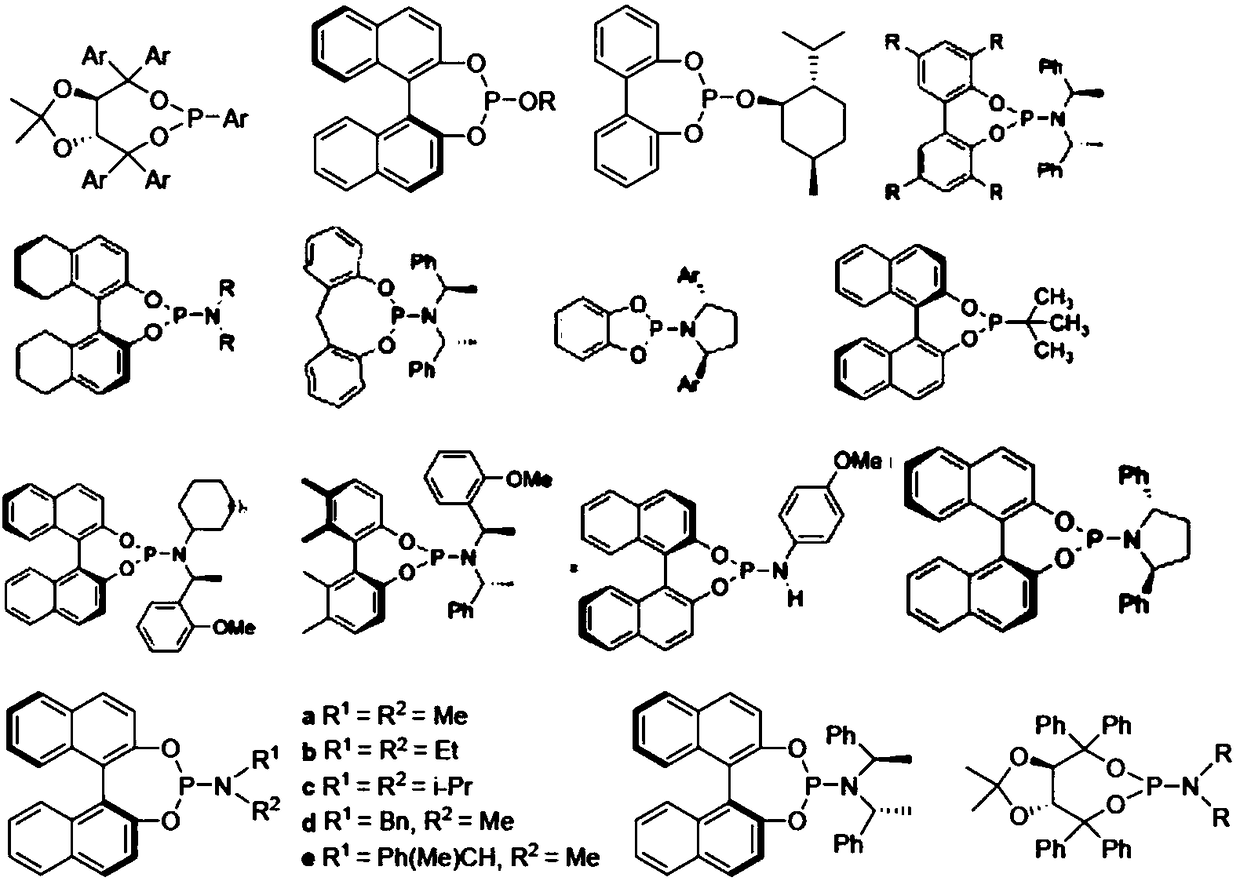
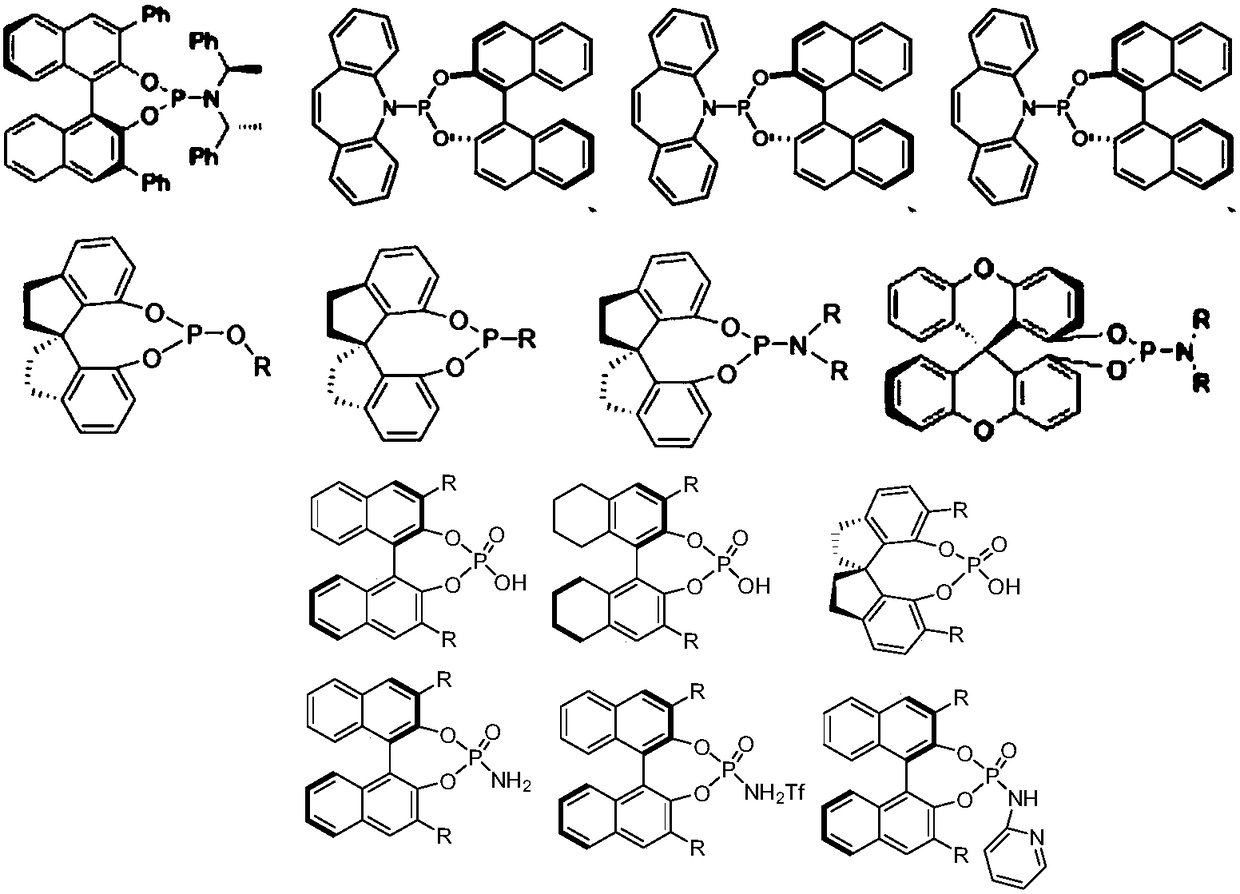
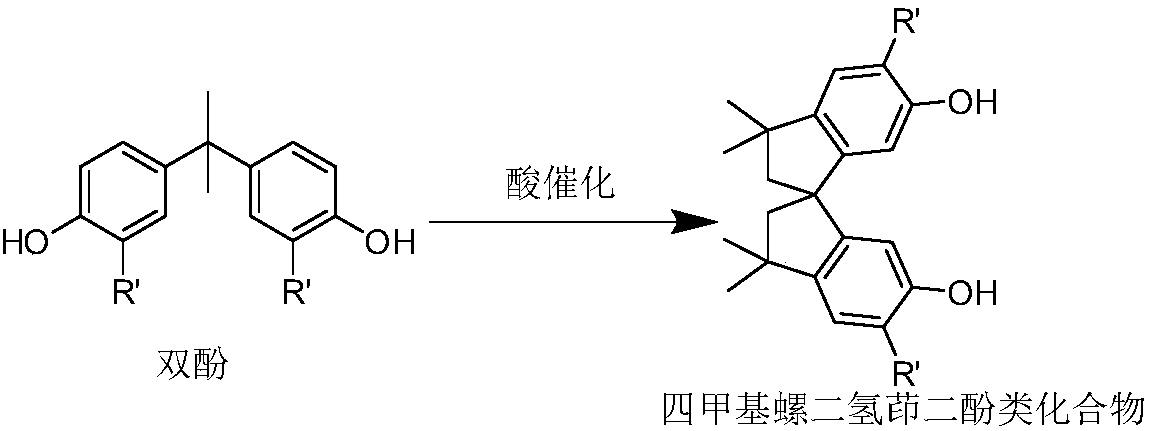
Monophosphine ligand based on tetramethyl spirobiindane skeleton, as well as intermediate, preparation method and application thereof
A technology of tetramethylspirodihydroindene and skeleton, applied in chemical instruments and methods, preparation of organic compounds, preparation of oximes, etc., can solve problems such as inability to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068]
[0069] Add 2g (6mmol) III-a racemic compound in the there-necked flask, add 150mL dichloromethane under nitrogen protection, cool in an ice bath, add 0.9mL trifluoroacetic acid (12mmol), add 4.28g m-chloroperoxybenzene in three times 27mmol of formic acid (m-CPBA) was stirred and dissolved, and the reaction solution was colorless and transparent. After stirring overnight at room temperature for 16 hours, the reaction solution turned light yellow. TLC plate monitoring reaction (with 2,4-dinitrophenylhydrazine color development), reaction finishes; Add 50 milliliters of sodium sulfite saturated solutions to quench the reaction, water successively, saturated sodium bicarbonate and saturated sodium chloride solution wash the organic phase, no Dry over sodium sulfate, concentrate to dryness under reduced pressure, add 10mL methanol to dissolve the residual solid, slowly add 20mL 3mol / L sodium hydroxide solution with stirring, react overnight at room temperature, monitor...
Embodiment 2
[0072]
[0073]Add 1.44g (R)-III-b compound (4mmol) in the there-necked flask, add 150mL dichloromethane under nitrogen protection, ice bath cooling, add 3.2g m-chloroperoxybenzoic acid (m-CPBA) 16mmol in three times, Add 0.6mL trifluoroacetic acid (8mmol), stir to dissolve, and the reaction solution is colorless and transparent. After stirring overnight at room temperature for 15 hours, the reaction solution turned light yellow. TLC plate monitoring reaction (with 2,4-dinitrophenylhydrazine color development), reaction finishes; Add 30 milliliters of sodium sulfite saturated solutions to quench the reaction, water successively, saturated sodium bicarbonate and saturated sodium chloride solution wash the organic phase, no Dry over sodium sulfate, concentrate to dryness under reduced pressure, add 40mL methanol to dissolve the residual solid, slowly add 16mL 1mol / L sodium hydroxide solution with stirring, react overnight at room temperature for 12 hours, monitor the reaction...
Embodiment 3
[0076]
[0077] According to the experimental procedure of Example 2, compound (R)-IIII-b was replaced with (R)-III-d to obtain product (R)-II-d (yield 92%). 1 H NMR (400MHz, CDCl 3 )δ6.55(s,2H),5.09(s,2H),3.71(s,6H),2.47(d,J=13.0Hz,2H),2.29(s,6H),2.22(d,J=13.0 Hz,2H),1.37(s,6H),1.31(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com