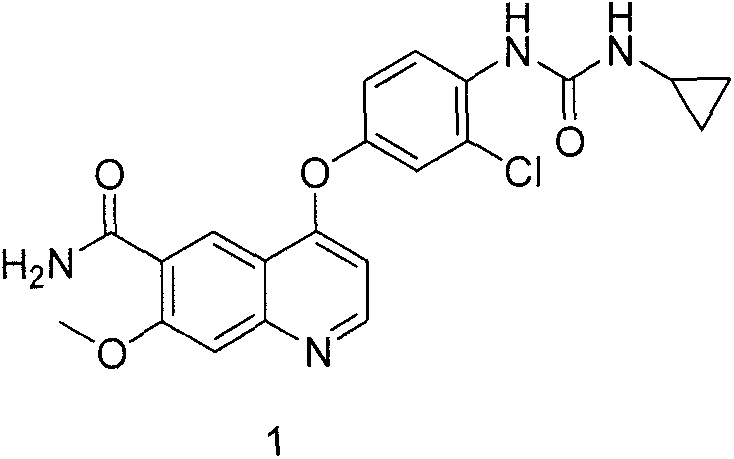
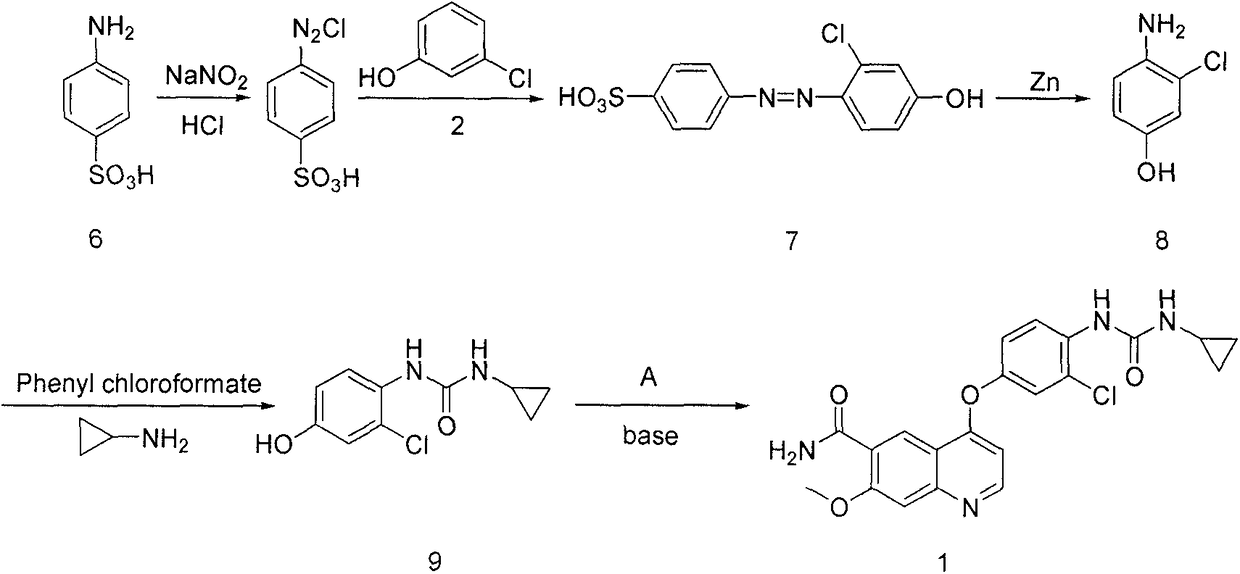
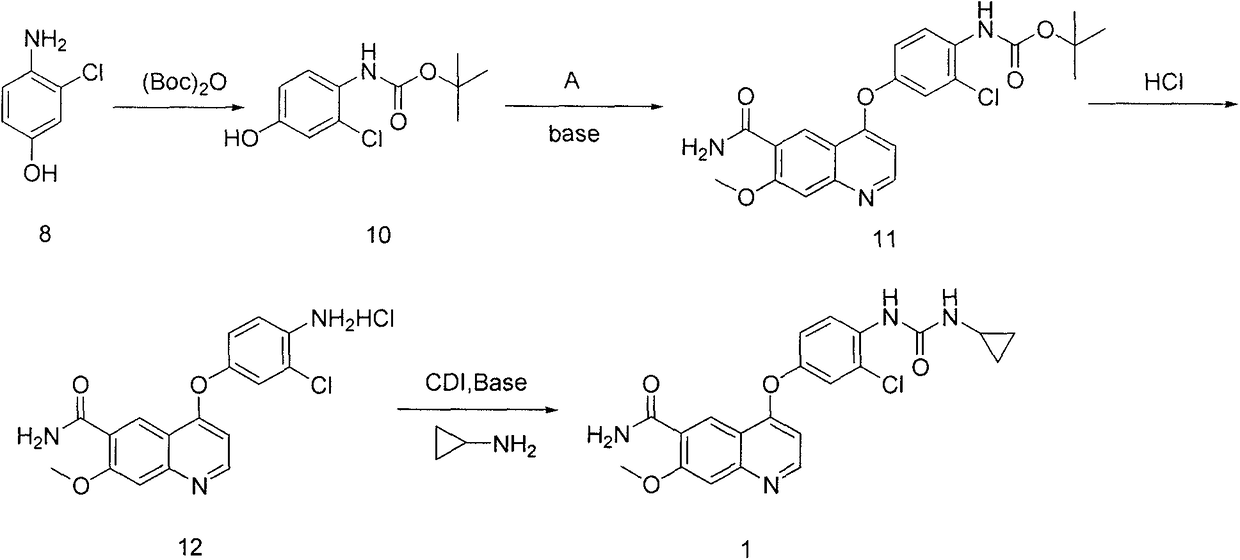
Synthesis method for lenvatinib
A synthesis method and technology of lenvatinib are applied in the field of synthesis route of thyroid cancer drug lenvatinib, which can solve the problems of low CDI activity, long synthesis steps, easy shedding of intermediates, etc., so as to avoid difficult to control low temperature and The use of diazonium salts, the effect of simplifying post-processing steps and shortening the synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Synthesis of 3-chloro-4-nitrophenol (3) according to literature (L-Y.Chen, et al, Arkivoc, 2014, 64-71, DOI: 10.3998 / ark.5550190.p008.587)
[0014]
Embodiment 2
[0016]
[0017] Dissolve 1.0 g of 3-chloro-4-nitrophenol (3), 1.42 g of 4-chloro-7-methoxyquinoline-6-amide (A) in 60 mL of dimethyl sulfoxide, and stir at 60°C for 36 hours , down to room temperature. The reaction solution was poured into 400mL of ice water, filtered, and the filter cake was washed with water and dried in vacuo to obtain a pale yellow solid 4 (1.14g, 52.9%).
[0018] [1HNMR (DMSO-d6) δ8.78(d, J=5.2Hz, 1H), 8.56(s, 1H), 8.25(d, J=9.0Hz, 1H), 7.88(s, 1H), 7.83(d , J=2.5Hz, 1H), 7.78(s, 1H), 7.57(s, 1H), 7.48(dd, J=9.0, 2.5Hz, 1H), 6.91(d, J=5.2Hz, 1H), 4.04 (s, 3H);
[0019] 13CNMR (DMSO-d6) δ165.71, 159.49, 158.22, 157.74, 153.46, 151.89, 144.29, 128.34, 127.60, 125.80, 124.39, 123.12, 119.84, 114.77, 108.11, 1065.795], 5
[0020] ESI-MS m / z: 374.2[m+H]+
Embodiment 3
[0022] Dissolve 1.0 g of 3-chloro-4-nitrophenol (3) and 1.70 g of 4-chloro-7-methoxyquinoline-6-amide (A) in 60 mL of chlorobenzene, stir at 120°C for 5 hours, drop to After reaching room temperature, filter, the filter cake was washed with water and toluene respectively, and then vacuum-dried to obtain light yellow solid 4 (1.50g, 69.6%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com