Benzocarbazole derivatives and organic light-emitting device thereof
A technology of benzocarbazole derivatives, applied in the field of benzocarbazole derivatives and organic electroluminescent devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
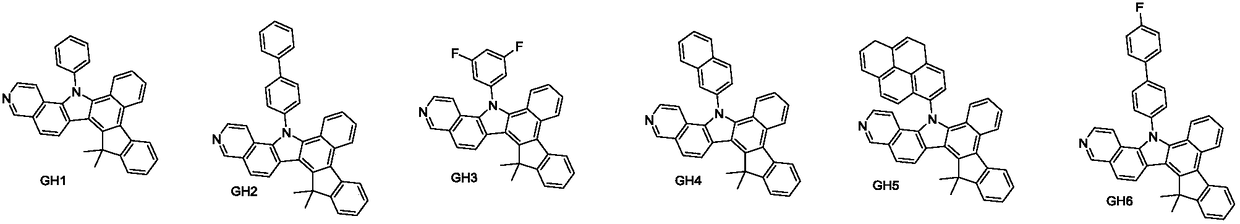
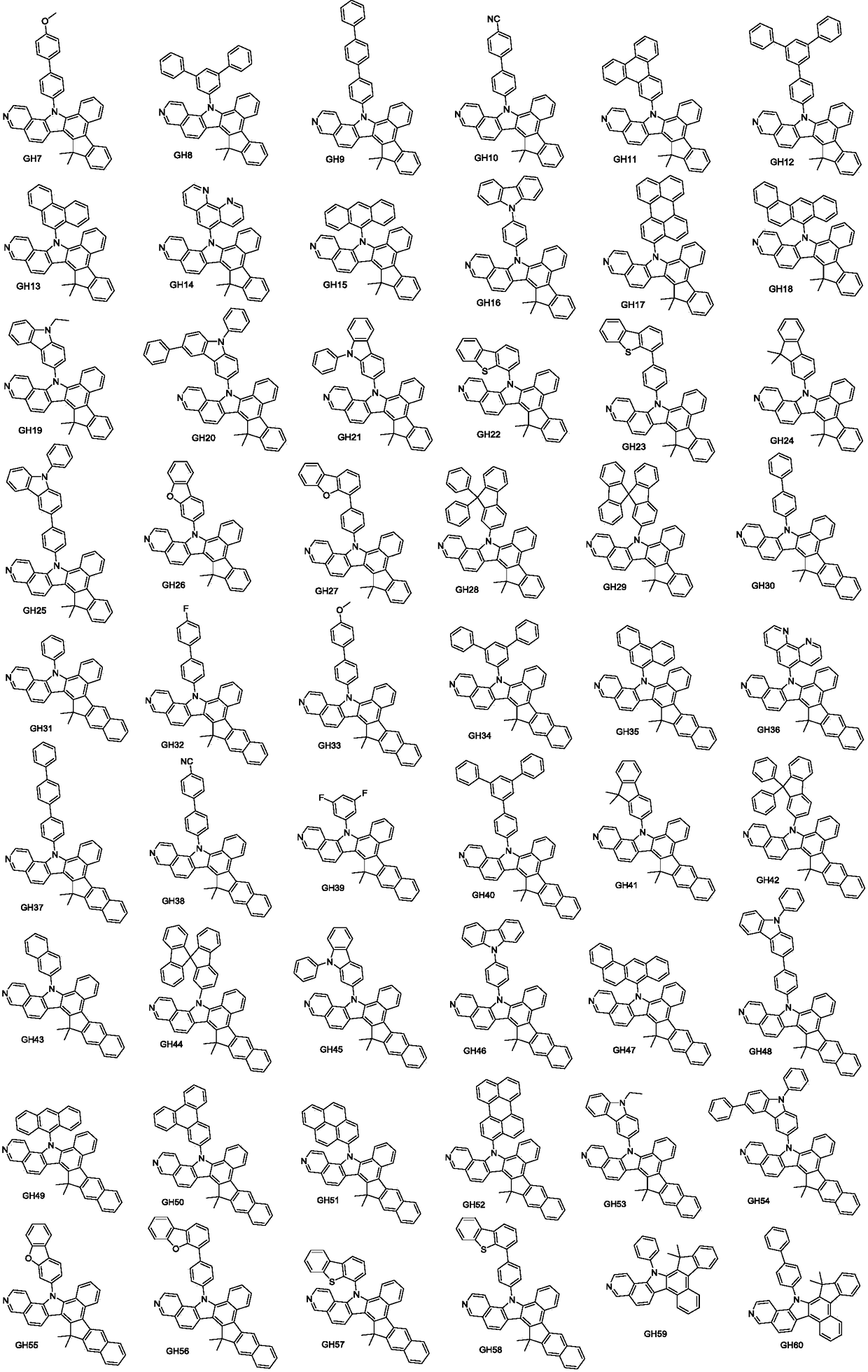
Examples
preparation example Construction
[0060] The preparation method of the benzocarbazole derivatives of the present invention, the specific synthetic route is as follows:
[0061]
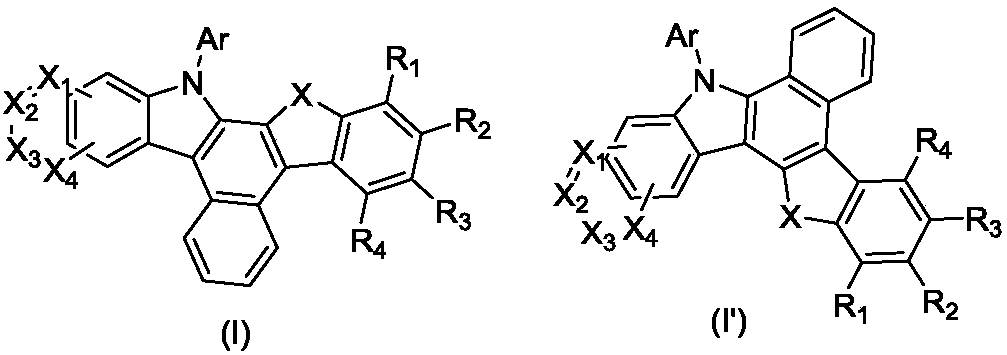
[0062] Wherein, X is selected from O, S or CY 1 Y 2 , Y 1 , Y 2 One of independently selected from substituted or unsubstituted C1-C4 aliphatic groups, substituted or unsubstituted C6-C12 aryl groups; X 1 、X 2 、X 3 、X 4 independently selected from CH or N; R 1 , R 2 , R 3 , R 4 independently selected from hydrogen, deuterium, halogen, cyano, C1-C12 aliphatic, substituted or unsubstituted C6-C60 aryl or substituted or unsubstituted C4-C60 heteroaryl; Ar is selected from substituted or Unsubstituted C6-C60 aryl or substituted or unsubstituted C4-C60 heteroaryl.
[0063] Using compound A as the starting material, first undergo Suzuki coupling reaction with boronic acid compound BH-1 or BH-2 to obtain intermediate B-1 or B-2; then undergo ring closure reaction to obtain intermediate C-1 or C- 2; react with a bromide contain...
Embodiment 1
[0069] Embodiment 1: the preparation of intermediate B
[0070]
[0071] Preparation of intermediate B-1: 15.12g (60mmol) 6-bromo-5-nitroisoquinoline, 16.91g (50mmol) 6-boronic acid-7,7-dimethylbenzo[C]fluorene, 4.99 g (4.32mmol) tetrakis (triphenylphosphine) palladium was added in 500ml of tetrahydrofuran, then 250ml of aqueous solution containing 12.44g (90mml) of potassium carbonate was added, and the reaction was refluxed for 24 hours. After the reaction, it was washed with water and methanol, and purified by column chromatography to obtain 18.33 g (44 mmol) of intermediate B-1 with a yield of 88%.
[0072] Intermediate B-2 to Intermediate B-16 can also be prepared by the above method.
Embodiment 2
[0073] Embodiment 2: the preparation of intermediate C
[0074]
[0075] Preparation of Intermediate C-1: Dissolve 12.49g (30mmol) of Intermediate B-1 in 150ml of o-dichlorobenzene, add 6.23g (37.5mmol) of triethyl phosphite, and react at 150°C for 24 hours. After cooling and suction filtration, the organic solvent in the filtrate was distilled off under reduced pressure, and the obtained residue was purified by column chromatography to obtain 12.94 g (26.7 mmol) of intermediate C-1 with a yield of 89%.
[0076] Intermediate C-2 to Intermediate C-16 can also be prepared by the above method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com