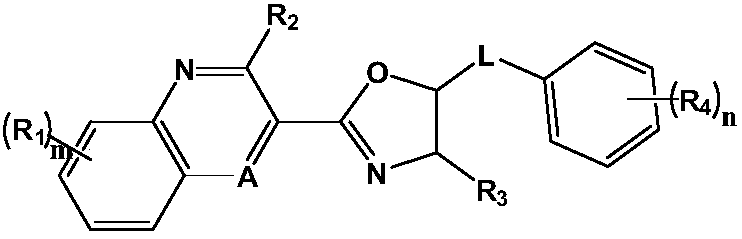
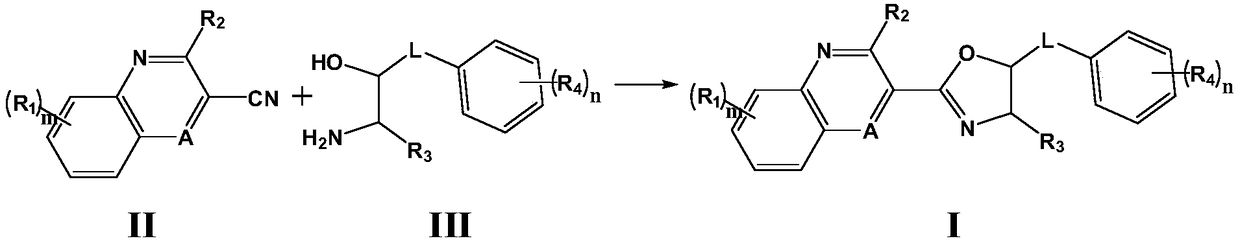
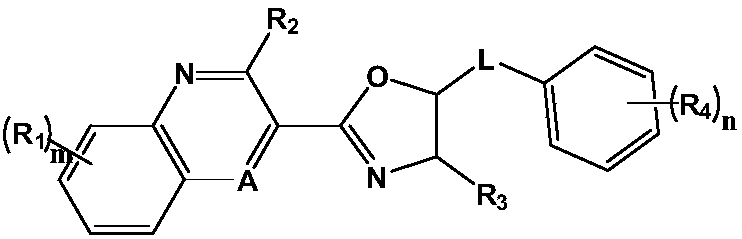
Drug for preventing and treating bronchogenic carcinoma and preparation method thereof
A drug and pharmacy technology, applied in the directions of drug combination, pharmaceutical formula, medical preparation containing active ingredients, etc., can solve the problem of difficult to cure lung cancer, and achieve the effect of good in vitro anti-tumor activity and outstanding inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Preparation of 4-((4-tert-butyl-2-(quinolin-3-yl)-4,5-dihydro (Azol-5-yl)methyl)phenol (compound 1)
[0074]
[0075] Put 5mmol quinoline-3-nitrile, 10mmol 4-(3-amino-2-hydroxy-4,4-dimethylpentyl)phenol, 0.5mmol sulfur, and 100ml toluene in a round bottom flask and heat under stirring To reflux, react for 10 hours. The reaction was monitored by TLC. After the reaction, it was cooled to room temperature, unreacted sulfur was filtered, and toluene was distilled off under reduced pressure. The residue was subjected to silica gel column chromatography using ethyl acetate / cyclohexane (1:3) as the mobile phase to obtain a white color. Solid 4-((4-tert-butyl-2-(quinolin-3-yl)-4,5-dihydro The azol-5-yl)methyl)phenol is 1.46 g, and the yield is 81.0%.
[0076] Mass spectrum (ESI): 360.18[M+H] +
[0077] Proton spectrum (400MHz, DMSO) δ9.63(s,1H),9.42(s,1H),8.94(s,1H),7.63-7.91(m,4H),7.15(d,2H),6.73(d, 2H), 3.62 (m, 1H), 2.89 (d, 2H), 1.54 (m, 1H), 0.96 (s, 9H).
Embodiment 2
[0078] Example 2: Preparation of 3-(5-(2,4-dichlorophenyl)-4-propyl-4,5-dihydro (Azol-2-yl)quinoline-7-amine (compound 2)
[0079]
[0080] Substitute 7-aminoquinoline-3-carbonitrile for quinoline-3-carbonitrile in Example 1, and substitute 3-amino-1-(2,4-dichlorophenyl)hexyl-2-ol for 4-(3 -Amino-2-hydroxy-4,4-dimethylpentyl)phenol, other operations are the same as in Example 1, to obtain 3-(5-(2,4-dichlorophenyl)-4-propyl-4, 5-dihydro (Azol-2-yl)quinoline-7-amine, the yield was 76.1%.
[0081] Mass spectrum (ESI): 414.11 [M+H] +
[0082] Proton spectrum (400MHz, DMSO) δ9.31(s,1H),8.89(s,1H),8.62(d,1H),7.94(s,1H),7.64(s,1H),7.42(s,1H) , 7.35(d, 1H), 7.16(d, 1H), 5.64(s, 2H), 3.65(m, 1H), 2.85(d, 2H), 1.53(m, 1H), 1.32(m, 2H), 1.23 (m, 2H), 0.91 (t, 3H).
Embodiment 3
[0083] Example 3: Preparation of 2-(5-fluoroquinoxalin-2-yl)-5-(4-(pyrrolidin-1-yl)benzyl)-4,5-dihydro Azole (compound 3)
[0084]
[0085] Use 5-fluoroquinoxaline-2-nitrile instead of quinoline-3-nitrile in Example 1, and use 1-amino-3-(4-(pyrrolidin-1-yl)phenyl)propane-2-ol Instead of 4-(3-amino-2-hydroxy-4,4-dimethylpentyl)phenol, other operations were the same as in Example 1, to obtain 2-(5-fluoroquinoxalin-2-yl)-5-( 4-(pyrrolidin-1-yl)benzyl)-4,5-dihydro Azole, the yield is 69.3%.
[0086] Mass spectrum (ESI): 377.17[M+H] +
[0087] Proton spectrum (400MHz, DMSO)δ8.75(s,1H), 7.62(d,1H), 7.53(t,1H), 7.30(d,1H), 7.14(d,2H), 6.74(d,2H) , 3.64 (m, 1H), 3.44 (t, 4H), 2.85 (d, 2H), 1.98 (t, 4H), 1.53 (d, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com