Recombinant herpes simplex virus and application thereof
A virus and chimeric virus technology, applied in the field of virology and tumor treatment, can solve the problems of replicating and killing tumor cells that have not yet been found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Embodiment 1. Construction of recombinant virus OVN and OVH
[0206] (1.1) Culture and titer determination of herpes simplex virus type I (HSV-1)
[0207] The wild-type HSV-1 virus strain KOS was purchased from ATCC Company of the United States (product number VR-1493 TM ), whose genome information has been published in NCBI (GenBank: JQ673480.1). Under the condition of MOI=0.1, the Vero cell (purchased from ATCC Company of the United States, product number CCL-81) was infected with the virus strain KOS TM ). After 48 hours, all cells were collected with a cell scraper and centrifuged to remove cell culture medium. The obtained cell pellet was resuspended in fresh complete medium and stored at -80°C. Subsequently, the cell suspension was repeatedly frozen and thawed (3 times), and then centrifuged to collect the supernatant to obtain the virus liquid. Aliquot the virus solution and store at -80°C.
[0208] Take 1×10 6 The density of the cells, the U-2 OS cells (p...
Embodiment 2
[0229] Example 2. Characterization of recombinant viruses OVN and OVH
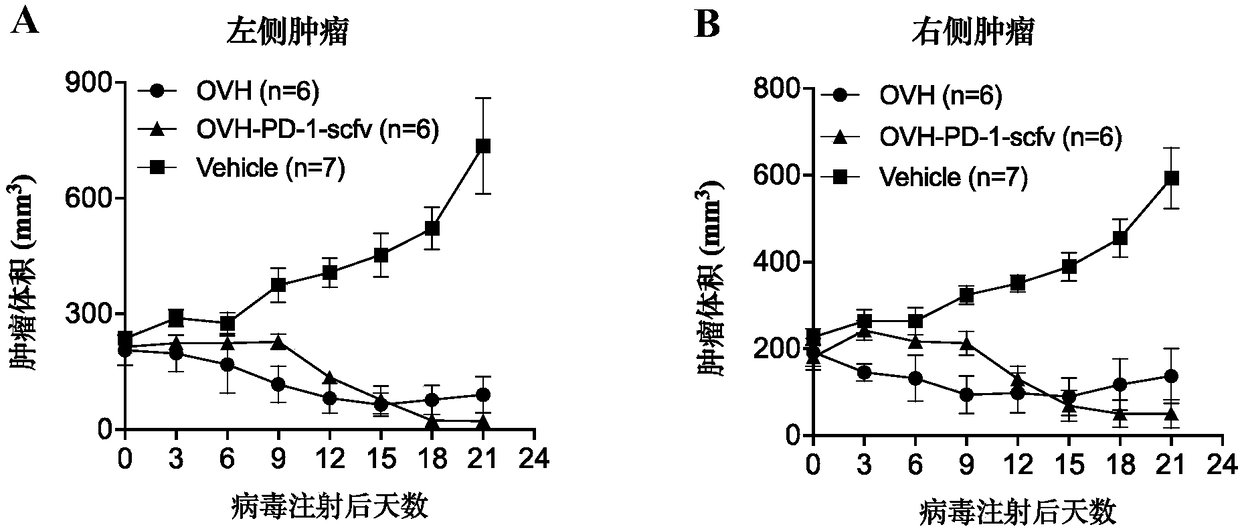
[0230] Referring to the method described in Example 1, a recombinant virus dICP0 was constructed, which, compared with the virus strain KOS, lacked two copies of the ICPO gene. Virus strain KOS and recombinant virus dICP0 were used as control viruses to characterize recombinant viruses OVN and OVH. image 3 Compared with the virus strain KOS, the genome modifications contained in the recombinant viruses OVN, OVH and dICP0 are schematically shown; among them, compared with the virus strain KOS, the recombinant virus dICP0 has lost two copies of the ICPO gene; the recombinant virus OVN has lost two copies. Copy of the ICP34.5 gene and ICPO gene; the recombinant virus OVN deleted the double copy of the ICP34.5 gene and the ICPO gene, and the native promoter of the ICP27 gene was replaced by the hTERT core promoter.
[0231] The gene deletion in the recombinant viruses OVN, OVH and dICP0 was verified by PCR m...
Embodiment 3
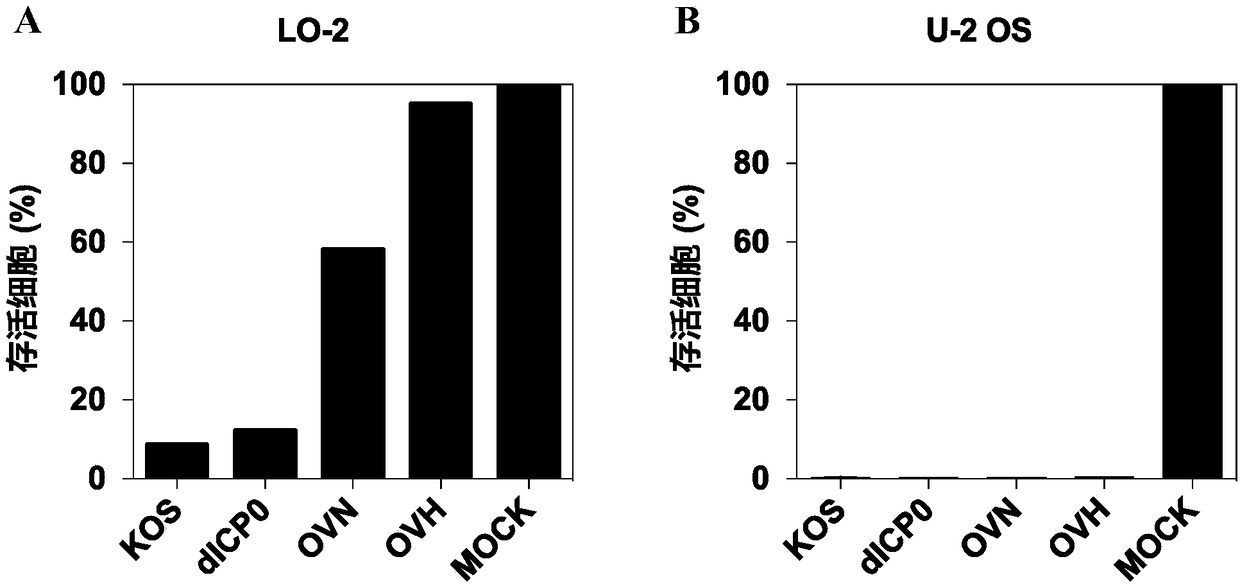
[0238] Example 3. Evaluation of Replication Ability and Killing Ability of Recombinant Virus OVN / OVH
[0239] Take 5-7.5×10 6Normal cells (L-O2 cells) and tumor cells (U-2 OS cells) in good condition and in the logarithmic growth phase were inoculated in a 6 cm culture plate. Subsequently, cultured cells were infected with viruses KOS, OVN, OVH or dICP0 at a multiplicity of infection of MOI=1. After 48 hours of infection, the state of the cells was observed under a microscope, and photographed and recorded. Subsequently, the virus-infected cells were digested, and the viability of the cells was calculated by trypan blue staining. Cell viability (%)=(number of living cells after virus infection) / (number of control cells not infected with virus)×100. Each group of experiments was set up to repeat in 3 wells, and the experimental results were the average of 3 independent experiments. In addition, referring to the protocol described in Example 1, virus titers at different time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com