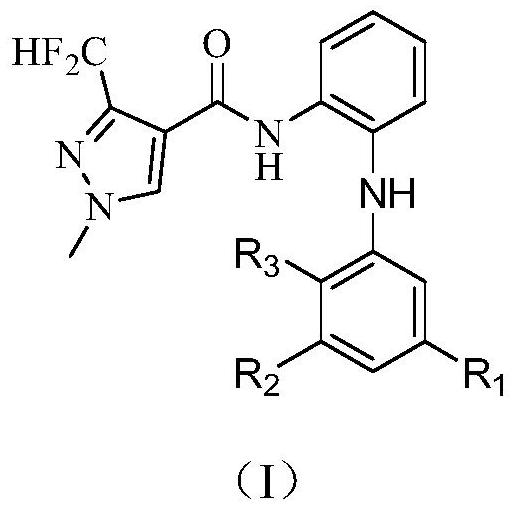
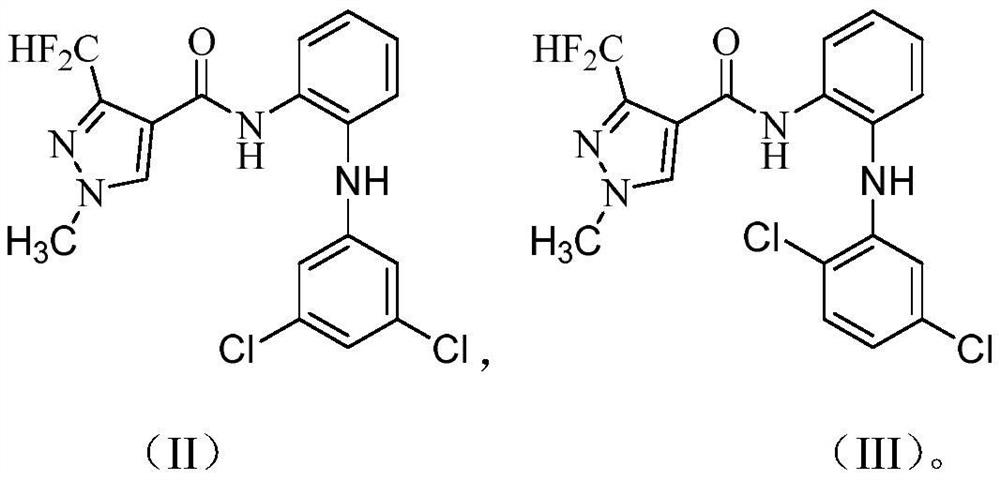
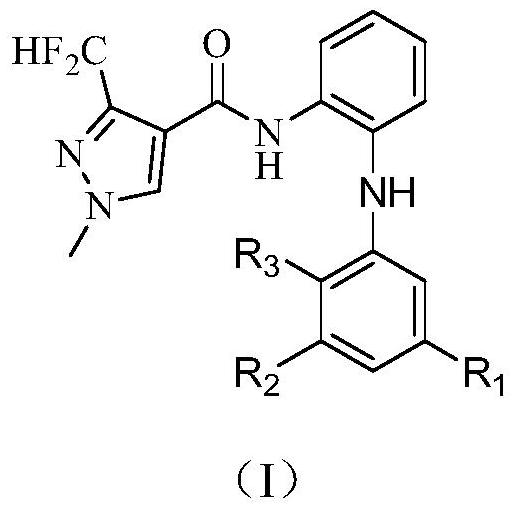
3-halogen diarylaminopyrazole amides and their application in pesticides
A technology of diarylaminopyrazole amides and compounds, which is applied in the field of 3-halogen diarylaminopyrazole amides, can solve the problems such as low concentration effect decline, achieve good effect, huge economic value, and good control effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of compound 1
[0044] For the synthesis method of pyrazole carboxamide, please refer to the reference (Xiao-Xiao Zhang et al, synthesis and biological evaluation of novel pyrazole carboxamide with diarylamine-modified scaffold as potent antifungal agents. Chinese Chemical Letters 28(2017) 1731–1736).
[0045] For the synthesis method of 3,5-dichloro-2-aminodiphenylamine, see reference (Xiao-Xiao Zhang et al, synthesis and biological evaluation of novel pyrazole carboxamide with diarylamine-modified scaffold as potent antifungal agents. Chinese Chemical Letters 28(2017) 1731 –1736)
[0046] Put 20mmol of 3,5-dichloro-2-aminodiphenylamine in a three-necked flask, then add 25mL of anhydrous dichloromethane and 5mL of triethylamine, stir in an ice bath, and then add 20mmol of pyrazole carboxylic acid chloride Transfer to a constant pressure funnel and slowly add it dropwise to a three-necked flask. After 30 minutes, the temperature was naturally raised to room...
Embodiment 2
[0050] The preparation method of compound 2 is the same as that of compound 1.
[0051] 1 H NMR (400MHz, DMSO-d 6): δ10.13(s,1H),8.41(s,1H),7.45(dd,J=7.9,1.5Hz,1H),7.41(s,1H),7.37(d,J=8.4Hz,1H) ,7.43–7.30(m,1H),7.36(t,J=54.0Hz,1H),7.33(s,1H),7.24(td,J=7.6,1.4Hz,1H),6.77(dd,J=8.4 ,2.4Hz,1H),6.74(d,J=2.4Hz,1H),3.95(s,3H);ESI-HRMS:m / z[M+H] + 411.0593.
[0052]
Embodiment 3
[0054] The preparation method of compound 3 is the same as that of compound 1.
[0055] 1 H NMR (400MHz, DMSO-d 6 ): δ9.58(s,1H),8.39(s,1H),7.55(s,1H),7.55(dd,J=8.0,1.2Hz,1H),7.44(t,J=54.0Hz,1H) ,7.22(dd,J=8.0,1.6Hz,1H),7.25(dd,J=5.2,3.9Hz,1H),7.13(dd,J=8.0,1.3Hz,1H),7.08(td,J=7.8 ,1.4Hz,1H),6.95(dd,J=6.4,2.8Hz,1H),6.88(d,1H),3.95(s,3H);ESI-HRMS:m / z[M+H] + 395.0886.
[0056]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com