A kind of preparation method of novel 5-allyl cyclopentenone and product thereof
A technology of allyl cyclopentenone and enynyl ester, applied in the field of preparation of novel 5-allyl cyclopentenone, which can solve the problems of poor selectivity, uncontrollable high yield of products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
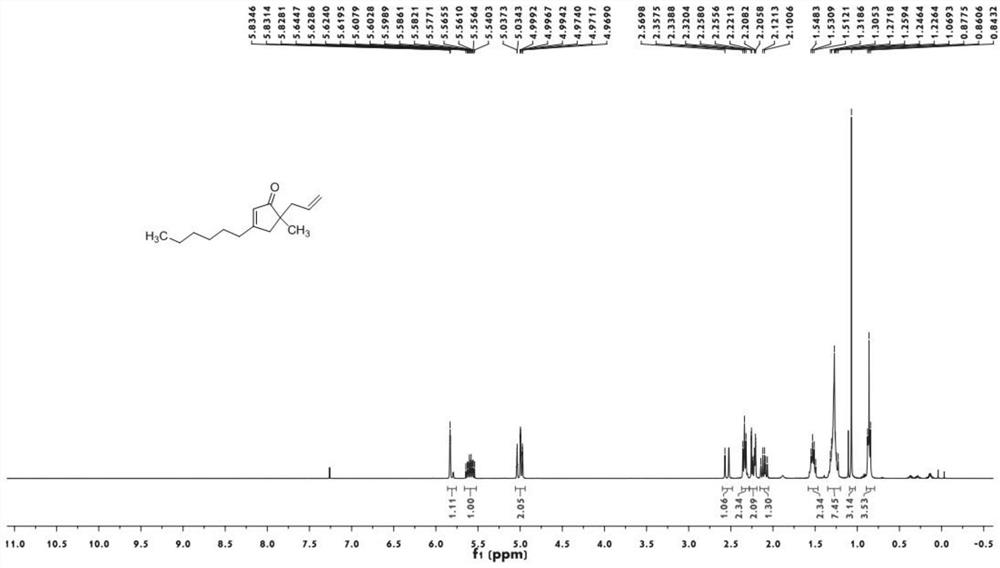
[0027]
[0028] Under nitrogen protection, add 25 mL of anhydrous THF to a 100 mL dry eggplant-shaped bottle, add 2-methyl-1-butene-3-yne (1.43 mL, 15 mmol), start stirring at -78 ° C, and add dropwise n-Butyllithium (6.00mL, 15mmol), add benzaldehyde (1.02mL, 10mmol) after 1.5h, stir for 2h, add allyl chloroformate (2.13mL, 15mmol), continue stirring for 30min, TLC monitors the reaction, the reaction After the end, it was quenched with saturated ammonium chloride solution, extracted with ethyl acetate (20mL*2), the organic phase was washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and rotary evaporated using a Heidolph rotary evaporator after suction filtration. The rotating speed is 200rpm, the temperature is 40°C, the vacuum degree is 0.06Mpa, and the processing time is 15min. Then, 1a (1.99g, 7.8mmol, yield 78%) was isolated by 200-300 mesh silica gel column chromatography, the eluent was ethyl acetate:petroleum ether=2:100.
[00...
Embodiment 2
[0033]
[0034] Under nitrogen protection, add bistriphenylphosphine palladium dichloride (140.3mg, 0.2mmol) and cuprous iodide (76.2mg, 0.4mmol) into a dry 100mL eggplant-shaped bottle, add 20mL THF, and start at room temperature Stir. Add 3 mL of diisopropylamine, then dissolve 1-phenyl-1-propynyl alcohol (1.32 g, 10 mmol) in 10 mL of THF into the reaction vessel, and finally add 6-iodo-1-phenyl-6-heptene- 1-Keto (4.71 g, 12 mmol), TLC monitored the reaction. After the reaction was completed, it was quenched with saturated ammonium chloride solution, extracted with ethyl acetate (20mL*2), the organic phase was washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and then evaporated using a Heidolph rotary evaporator after suction filtration. The rotating speed is 200rpm, the temperature is 40°C, the vacuum degree is 0.06Mpa, and the processing time is 10min. Then through 200-300 mesh silica gel column chromatography, the eluent is ethy...
Embodiment 3
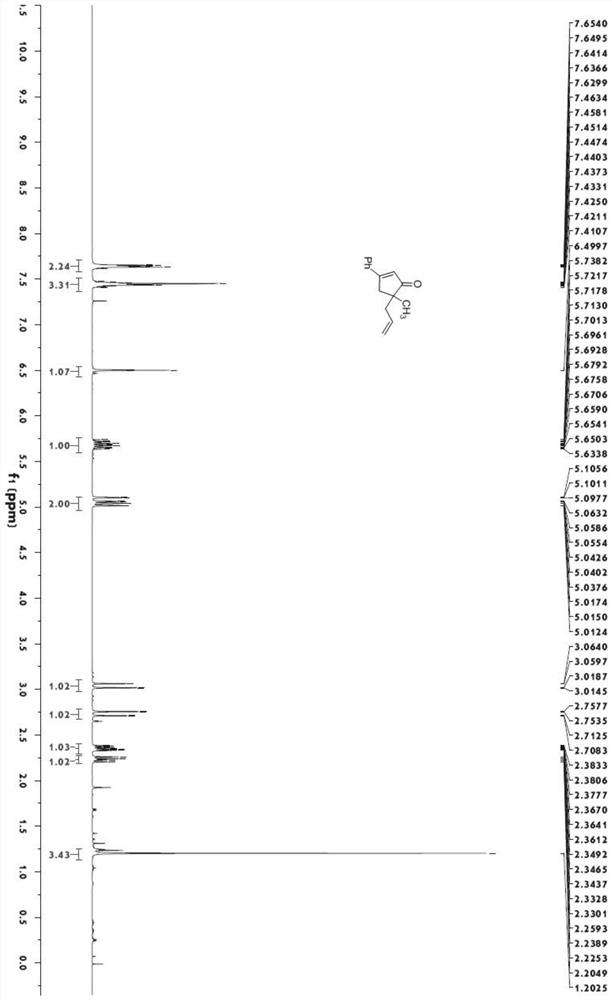
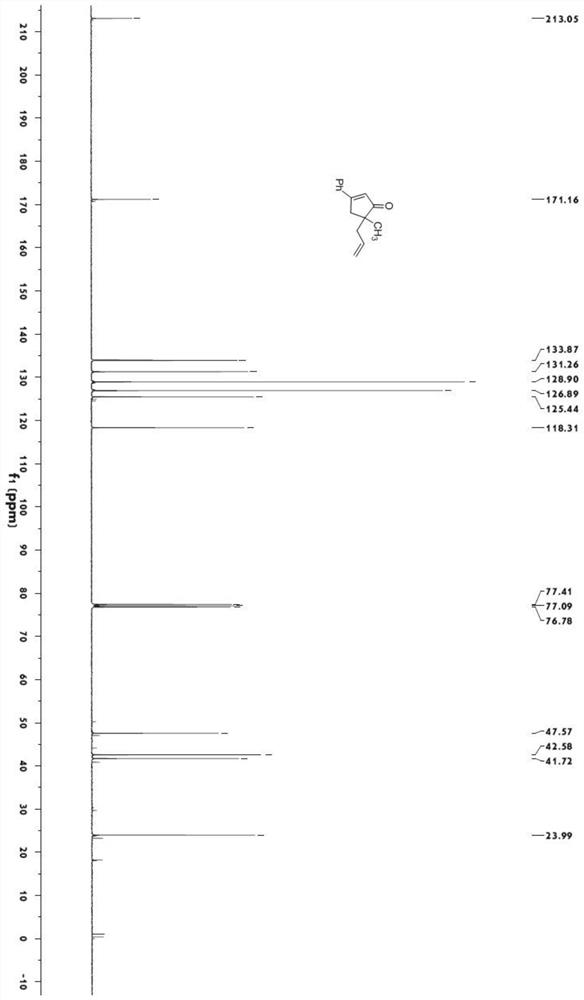
[0040]
[0041] Under nitrogen protection, add bistriphenylphosphine palladium dichloride (140.3mg, 0.2mmol) and cuprous iodide (76.2mg, 0.4mmol) into a dry 100mL eggplant-shaped bottle, add 20mLTHF, and start stirring at room temperature . Add 3 mL of diisopropylamine, then dissolve 1-phenyl-1-propynyl alcohol (1.32 g, 10 mmol) in 10 mL of THF and add to the reaction vessel, and finally add iodide (N-(4-iodopent-4-en-1 -yl)-4-methyl-N-phenylbenzenesulfonamide) (5.29g, 12mmol), TLC monitored the reaction. After the reaction was completed, it was quenched with saturated ammonium chloride solution, extracted with ethyl acetate (20mL*2), the organic phase was washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and then evaporated using a Heidolph rotary evaporator after suction filtration. The rotating speed is 200rpm, the temperature is 40°C, the vacuum degree is 0.06Mpa, and the processing time is 10min. Then through 200~300 mesh silica ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com