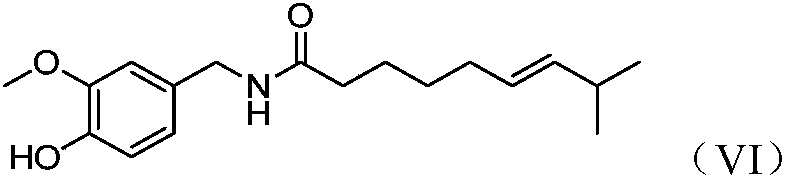
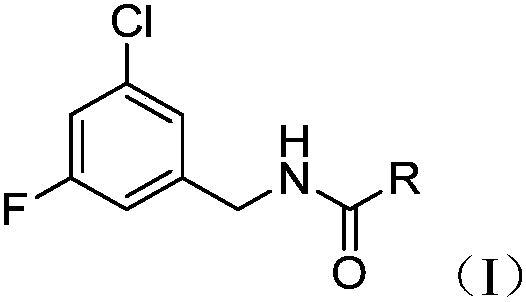
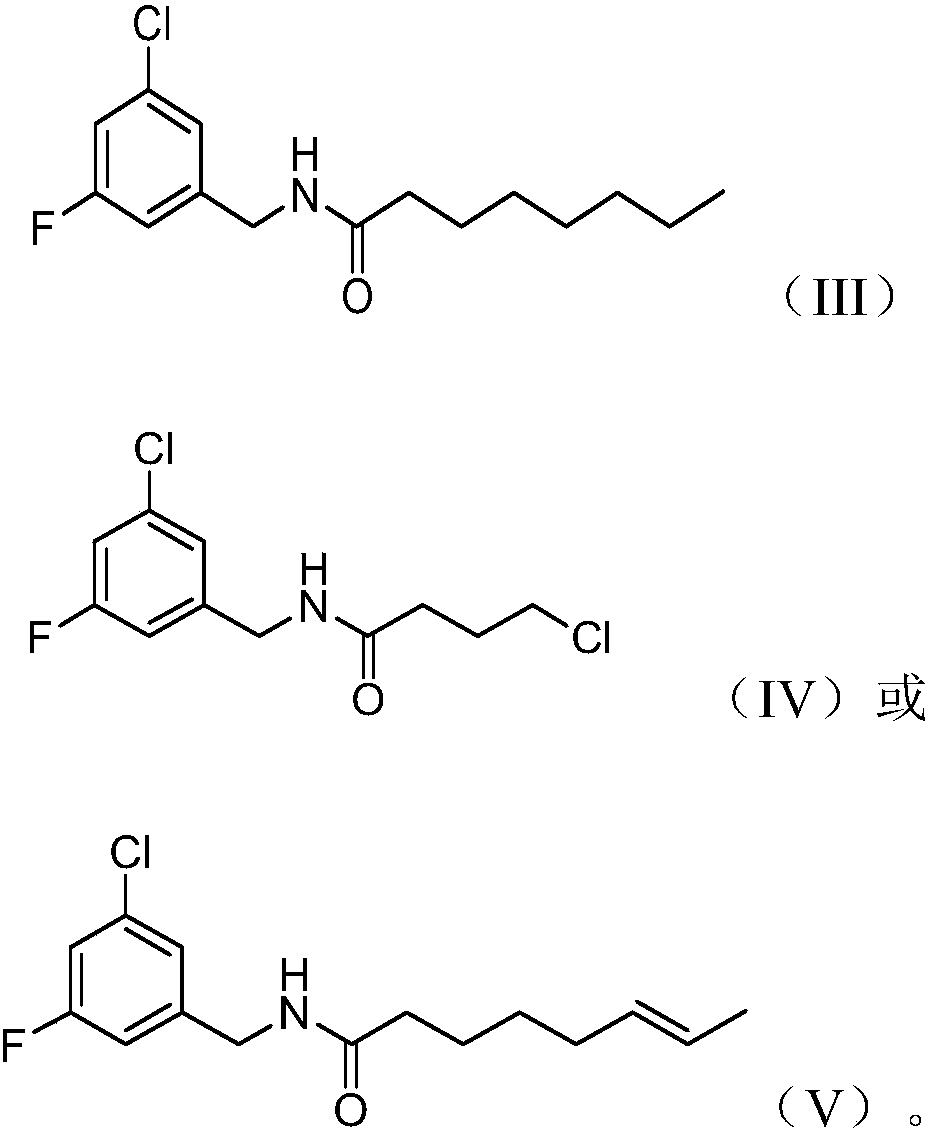
Synthesis and application of capsaicin halogenated derivative
A technology of reactions and compounds, applied in the field of chemistry, can solve problems such as high price and limited supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The preparation of compound as described in general formula (I) comprises the following steps:
[0047]
[0048] 3-Chloro-5-fluorobenzylamine reacts with formula (II) compound acid chloride to obtain formula (I) compound, and the molar ratio of 3-chloro-5-fluorobenzylamine and formula (II) compound acid chloride is 1:1.1-1.5 , R is C 1-9 Alkyl, halogen substituted C 1-9 Alkyl, C 1-9 Alkenyl, halogen substituted C 1-9 Alkenyl.
[0049] The preparation of the compound described in general formula (I) has one or more features selected from the following:
[0050] (i) the 3-chloro-5-fluorobenzylamine is reacted with the compound acid chloride of formula (II) in an organic solvent, and the organic solvent is selected from the group consisting of dichloromethane, tetrahydrofuran or pyridine;
[0051] (ii) the reaction is carried out in the presence of a base, and the base is selected from the group consisting of triethylamine, pyridine;
[0052] The reaction of (iii)...
Embodiment 1
[0073] Synthesis of N-(3-chloro-5-fluorobenzyl)octylamide
[0074]
[0075] Weigh 159mg of 3-chloro-5-fluorobenzylamine (1mmol) into a dry 50mL eggplant-shaped reaction bottle, add 15mL of dichloromethane, stir until the substrate is completely dissolved, add 200μL of triethylamine and heat at 0~5℃ A solution of 194 mg octanoyl chloride (1.2 mmol dissolved in an appropriate amount of dry dichloromethane) was slowly added dropwise in an ice bath. After the dropwise addition was completed, the mixture was followed and monitored by TLC at room temperature. When the raw material point disappeared, it was washed three times with 20 mL of saturated sodium bicarbonate, water and saturated NaCl aqueous solution, and dried over anhydrous sodium sulfate. After rotary evaporation under reduced pressure, silica gel column chromatography separated to obtain N-(3-chloro-5-fluorobenzyl)octylamide. 1 H NMR (CDCl 3 , 500MHz), δ: 8.19 (1H, s, -NH), 7.62 (1H, d, J = 2.0Hz), 7.32 (1H, s), 6....
Embodiment 2
[0077] Synthesis of 4-chloro-N-(3-chloro-5-fluorobenzyl)butanamide
[0078]
[0079] Weigh 159mg of 3-chloro-5-fluorobenzylamine (1mmol) into a dry 50mL eggplant-shaped reaction bottle, add 15mL of dichloromethane, stir until the substrate is completely dissolved, add 200μL of triethylamine and heat at 0~5℃ A solution of 168 mg of chlorobutyryl chloride (1.2 mmol dissolved in an appropriate amount of dry dichloromethane) was slowly added dropwise in an ice bath. After the dropwise addition was completed, the mixture was followed and monitored by TLC at room temperature. When the raw material point disappeared, it was washed three times with 20 mL of saturated sodium bicarbonate, water and saturated NaCl aqueous solution, and dried over anhydrous sodium sulfate. After rotary evaporation under reduced pressure, it was separated by silica gel column chromatography to obtain the synthesis of 4-chloro-N-(3-chloro-5-fluorobenzyl)butyramide. 1 H NMR (CDCl 3 , 500MHz), δ: 8.19 (1...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap