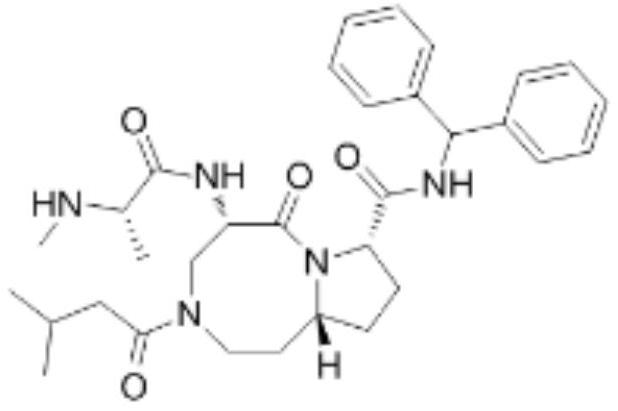
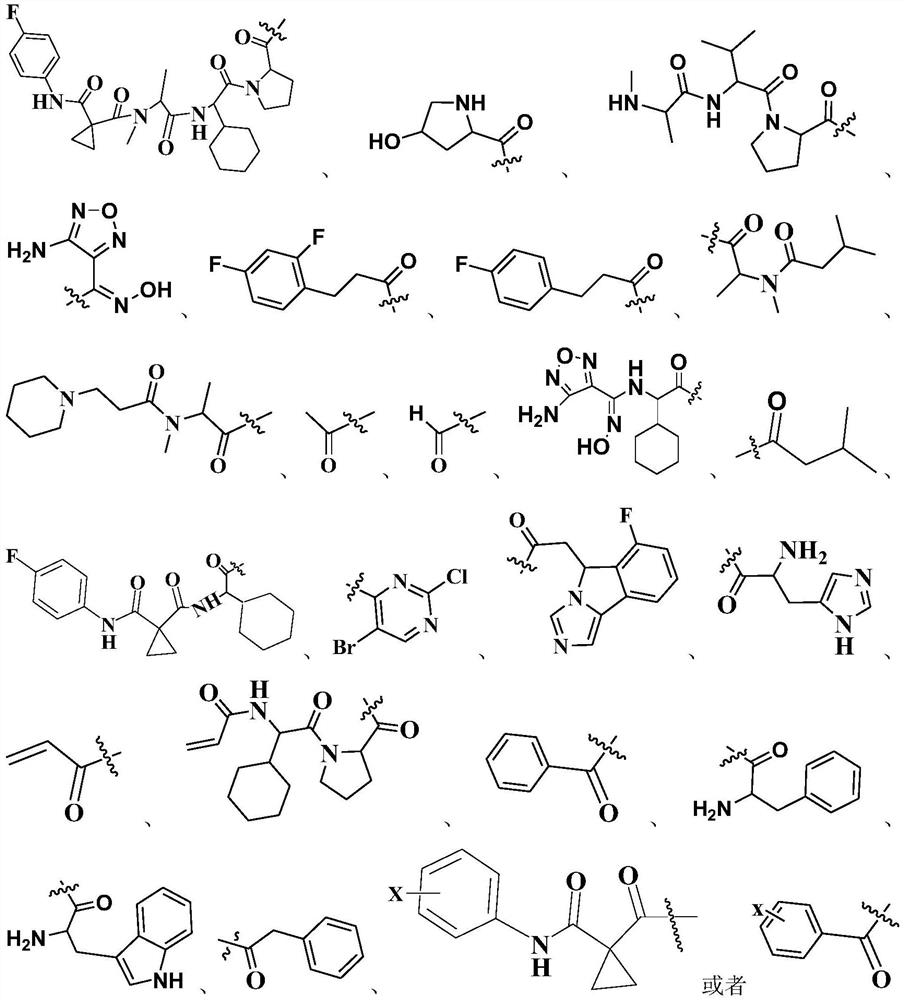
An anti-tumor apoptosis protein inhibitor
A compound, pharmaceutical technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062]
[0063] Step 1: Dissolve 12.73g of diphenylmethylamine in 200ml of DMF, add 40.69g of Fmoc-Cys(Trt)-OH, 39.49g of HBTU and 13.47g of DIEA in sequence, stir at room temperature for 2h, and detect by TLC (PE:EA=2: 1) until the raw material reacts completely. Add 20ml of piperidine to the reaction solution, stir the reaction at room temperature, and check by TLC (PE:EA=2:1) until the reaction of the raw materials is complete. The reaction solution was poured into 1L of water, extracted twice with ethyl acetate (500ml*2), the organic phases were combined, washed once with saturated sodium chloride, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure. The residue was purified by column chromatography (eluent DCM:MeOH) to obtain compound I-1-1 (30.02g yellow oily liquid, [M+H] + = 529.7);
[0064] Step 2: Dissolve 30.00g of compound I-1-1 in 150ml of DMF, add 19.15g of Fmoc-proline, 32.26g of HBTU and 11.00g of DIEA in sequence, sti...
Embodiment 2
[0072]
[0073]Step 1: Dissolve 1.40g of compound I-1-6 in 40ml of DCM, add 8ml of trifluoroacetic acid, and react at room temperature for 2.5h. After the reaction, adjust the pH to 7-8 with saturated aqueous sodium bicarbonate solution, separate the liquid, and use it for the aqueous phase 40ml of DCM was stripped once, and the organic phases were combined. The organic phase was washed once with saturated sodium chloride, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure. Add 30ml of tertiary methyl ether and beat at room temperature for 0.5h, and filter to obtain compound I-2-1 (2.41g, white solid);
[0074] Step 2: Dissolve 1.0 g of compound I-2-1 in 40 ml of DMF, add 0.44 g of N-Boc-2-methylalanine, 1.22 g of HBTU and 0.55 g of DIEA in sequence, react at room temperature for 1.0 h, and detect by TLC (DCM: MeOH=15:1), until the reaction of raw materials is complete. Add 8ml of piperidine to the reaction solution, react at room tempe...
Embodiment 3
[0078]
[0079] Step 1: Dissolve 0.21g of compound I-2-2 in 10ml of DMF, add 0.2g of compound 2, and react at room temperature for 1.5h. After the reaction is over. Pour the reaction solution into 100ml of water, extract 40ml*2 with ethyl acetate, combine the organic phases, wash the organic phase once with saturated sodium chloride, dry over anhydrous sodium sulfate, concentrate to dryness under reduced pressure, and perform column chromatography (eluent DCM : MeOH) purified to give compound I-3 (0.15g, white solid, [M+H] + = 1021.7).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com