Hexamethyl six-membered cucurbit ring supramolecular self-assembly carrier and its application
A technology of self-assembly of hexamethyl-hexamelon and supramolecules, which is applied in organic chemistry, luminescent materials, chemical instruments and methods, etc., can solve the problems of limited development and application, complex preparation process, high processing cost, etc., and achieve the preparation method Convenience and quickness, wide application prospects, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
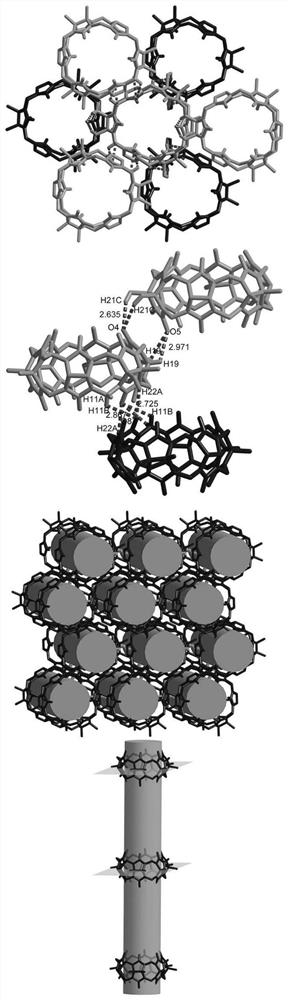
[0036] 1. Hexamethyl six-membered melon ring supramolecular self-assembly carrier, the structure diagram is as follows figure 1 shown; its molecular formula is:
[0037] C 42 h 106 N 24 o 41 . Its crystal data and structural details are shown in Table 1.
[0038] Table 1 Crystal data of the hexamethyl six-membered cucurbit ring supramolecular self-assembly carrier of the present invention
[0039]
[0040] 2. Preparation of hexamethyl six-membered cucurbit ring supramolecular self-assembly carrier: add hexamethyl six-membered cucurbit ring to neutral water, oscillate to make it dissolve, and obtain the product.
Embodiment 2
[0042] Production of red solid fluorescent material: Add hexamethyl hexa-membered cucurbit ring (0.126g, 0.1mmol) into 10mL neutral water, shake to dissolve, and obtain colorless crystals in neutral water within 5 days to obtain hexamethyl Six-membered melon ring supramolecular self-assembly carrier, the yield is 0.102g (81%);
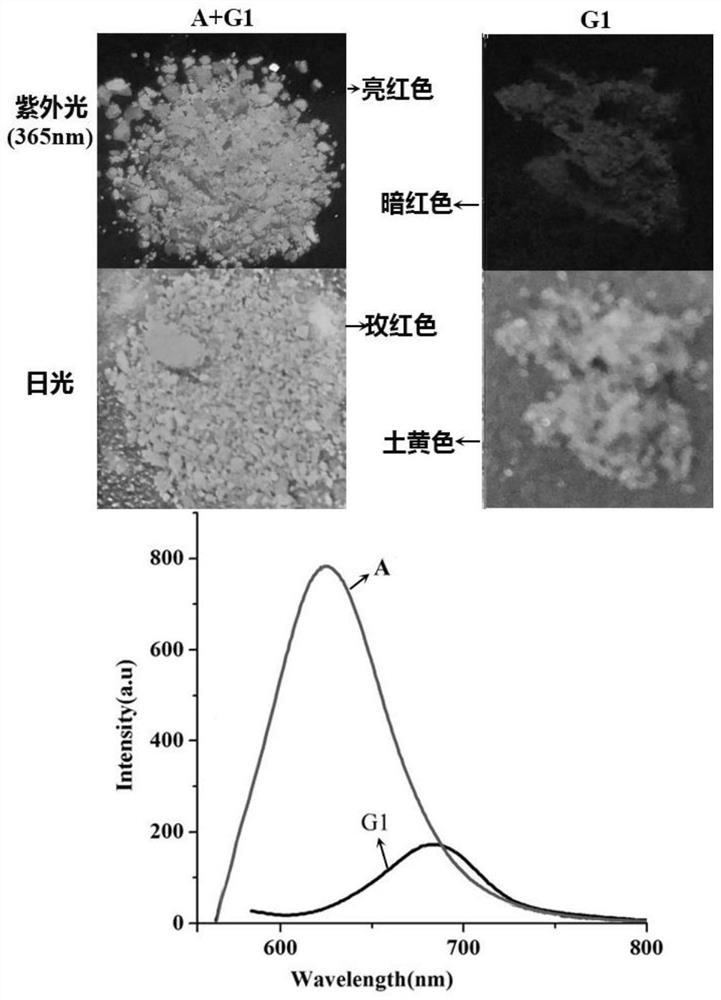
[0043] The above-mentioned hexamethyl six-membered cucurbit ring supramolecular self-assembly carrier (10.0 mg) was added to 0.5 mL to a concentration of 5 × 10 -3 M's Rhodamine B in acetonitrile solution, shaking, and filtering to obtain a red solid fluorescent material. The molecular structural formula of the rhodamine B and its color change under sunlight and ultraviolet light are as follows: figure 2 shown.
Embodiment 3
[0045] Production of green fluorescent solid material: Add hexamethyl hexa-membered cucurbit ring (0.126g, 0.1mmol) into 10mL neutral water, shake to dissolve, and obtain colorless crystals in neutral water within 5 days to obtain hexamethyl Six-membered melon ring supramolecular self-assembly carrier, the yield is 0.102g (81%);
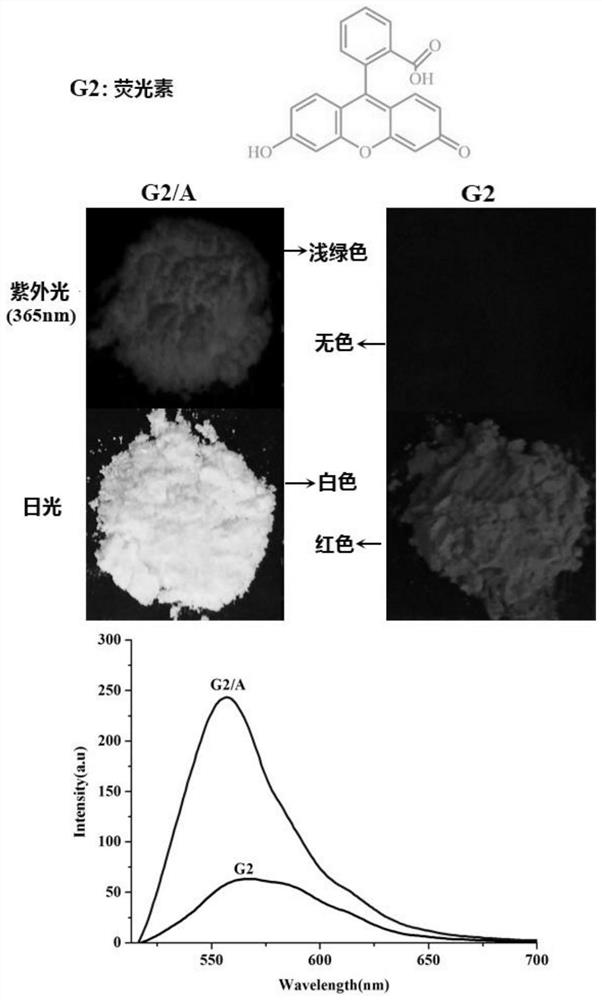
[0046]The above-mentioned hexamethyl six-membered cucurbit ring supramolecular self-assembly carrier (10.0 mg) was added to 0.5 mL to a concentration of 5 × 10 -3 M in the acetonitrile solution of fluorescein, shaken, and filtered to obtain a green solid fluorescent material. The molecular structural formula of the fluorescein and its color change under sunlight and ultraviolet light are as follows: image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com