Benzoxazine resin monomer based on magnolia cortex derivative and preparation method and application thereof
A technology of benzoxazine and resin monomer, applied in the field of benzoxazine resin monomer and its preparation, can solve the problems of low relative molecular weight of polymer, low elongation at break, limited application scope, etc. The effect of simple implementation, preparation and oil resource saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
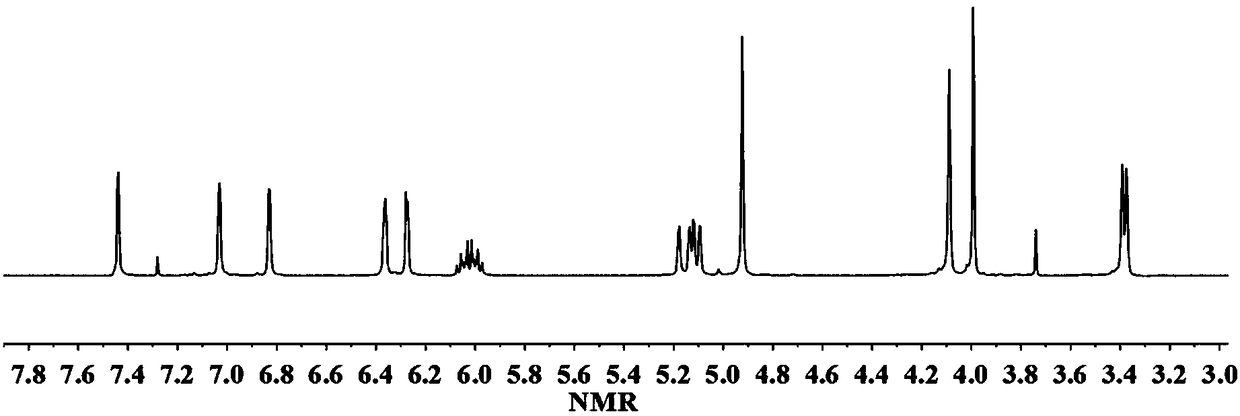
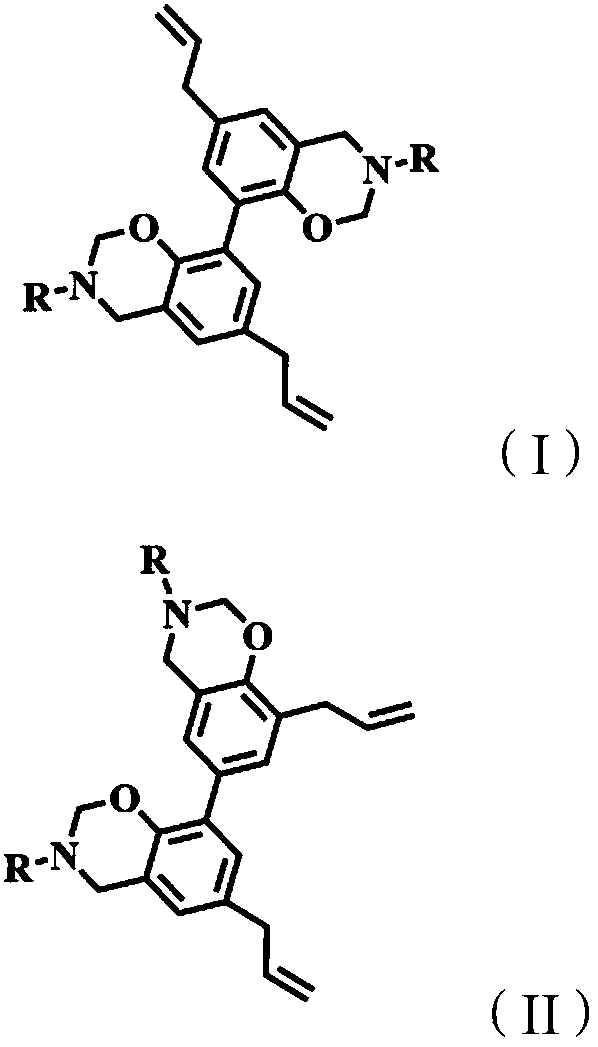
[0030] Dissolve 1 mol of magnolol, 2 mol of furfurylamine and 6 mol of paraformaldehyde in a mixed solution of 500 mL of dioxane and toluene (the ratio of dioxane to toluene is 2:3), and react at 90°C for 32 hour, the mixed solvent was removed by rotary evaporation under reduced pressure, after washing and drying, the magnolol furfurylamine benzoxazine with a structure as shown in formula (I-1) was obtained, and the productive rate was 91.5%. 1 H-NMR such as figure 1 As shown, each peak on the figure is in one-to-one correspondence with the hydrogen atoms on the structure of the magnolol furfurylamine benzoxazine compound.
[0031]
[0032] The magnolol furfurylamine benzoxazine is heated to 260°C in a blast oven for curing to obtain a cured product magnolol furfurylamine polybenzoxazine. The glass transition of the obtained cured product is 340°C, and the storage modulus in the rubber state is 1800Mpa, T d10 It is 467°C.
Embodiment 2
[0034] (1) Dissolve the paraformaldehyde of 1mol honokiol, 2mol furfurylamine and 7.5mol in the mixed solution of 650mL dioxane and toluene (the ratio of dioxane and toluene is 3:1), in React at 100°C for 52 hours, remove the mixed solvent by rotary evaporation under reduced pressure, wash with water and dry to obtain honokiol furfurylamine benzoxazine with the structure shown in formula (II-1), with a yield of 90.8%. H NMR spectrum 1 H-NMR such as figure 2 As shown, each peak on the figure is in one-to-one correspondence with the hydrogen atoms on the honokiol furfurylamine benzoxazine compound structure.
[0035]
[0036] The honokiol furfurylamine benzoxazine is heated to 260° C. for curing in a blast oven to obtain a cured product, the honokiol furfurylamine polybenzoxazine. The glass transition of the obtained cured product is 360°C, and the storage modulus in the rubber state is 1900Mpa, T d10 It is 476°C.
Embodiment 3
[0038] (1) The paraformaldehyde of 1mol honokiol, 2mol ethylamine and 6.1mol is dissolved in the mixed solution of 550mL dioxane and toluene (the ratio of dioxane and toluene is 4:1), in React at 120°C for 24 hours, remove the mixed solvent by rotary evaporation under reduced pressure, wash with water and dry to obtain honokiol ethylamine benzoxazine with the structure shown in formula (II-2), with a yield of 95.5%.
[0039]
[0040] The honokiol ethylamine benzoxazine is heated to 260° C. and solidified in a blast oven to obtain the cured product honokiol ethylamine polybenzoxazine. The glass transition of the obtained cured product is 270°C, and the storage modulus in the rubber state is 1600Mpa, T d10 It is 441°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition | aaaaa | aaaaa |
| Storage modulus | aaaaa | aaaaa |
| Glass transition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com