Response-guided hcv therapy
A therapy and dosage form technology, applied in the field of providing response-guided HCV therapy, can solve problems such as high cost and difficulty in patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
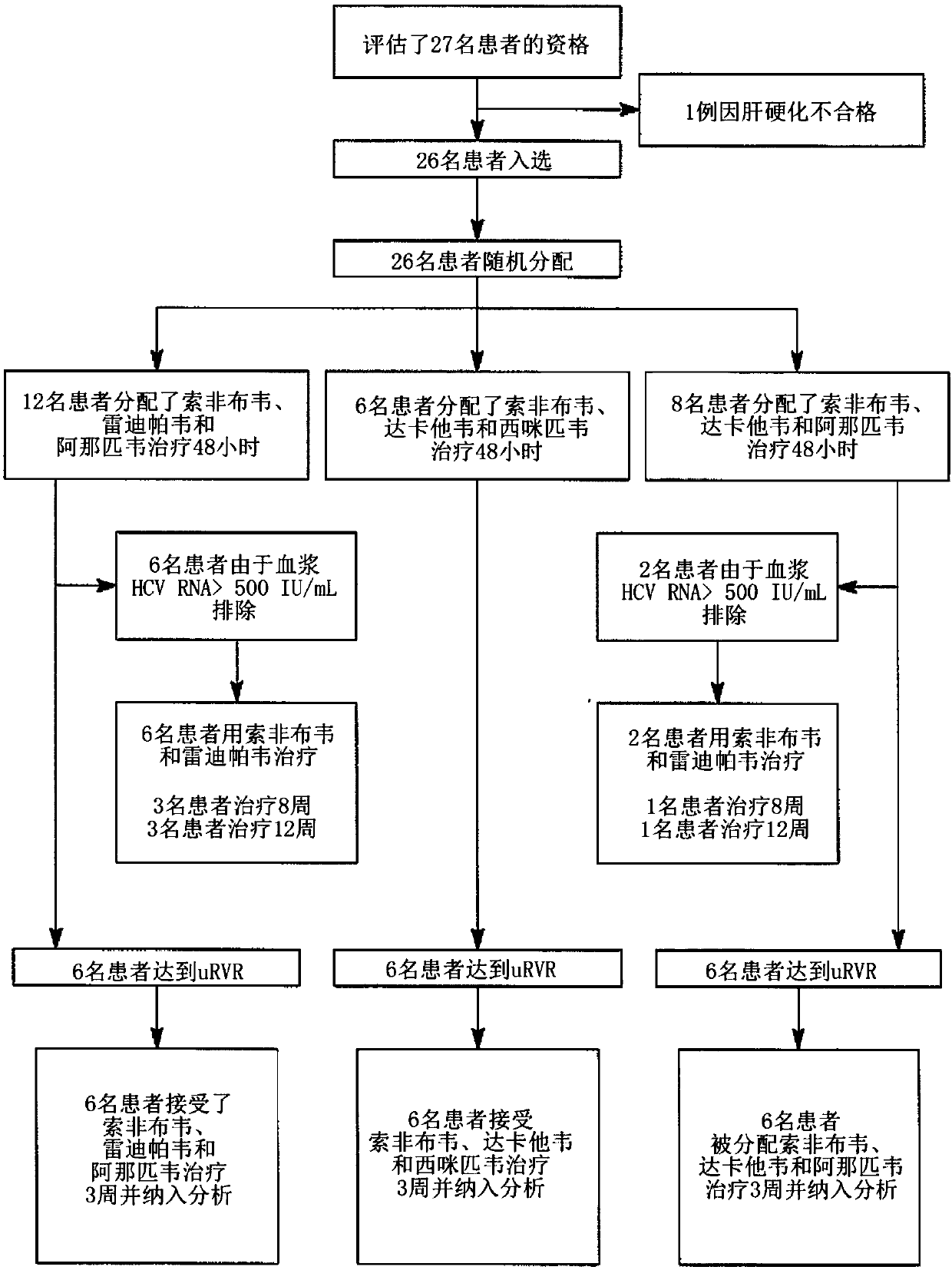
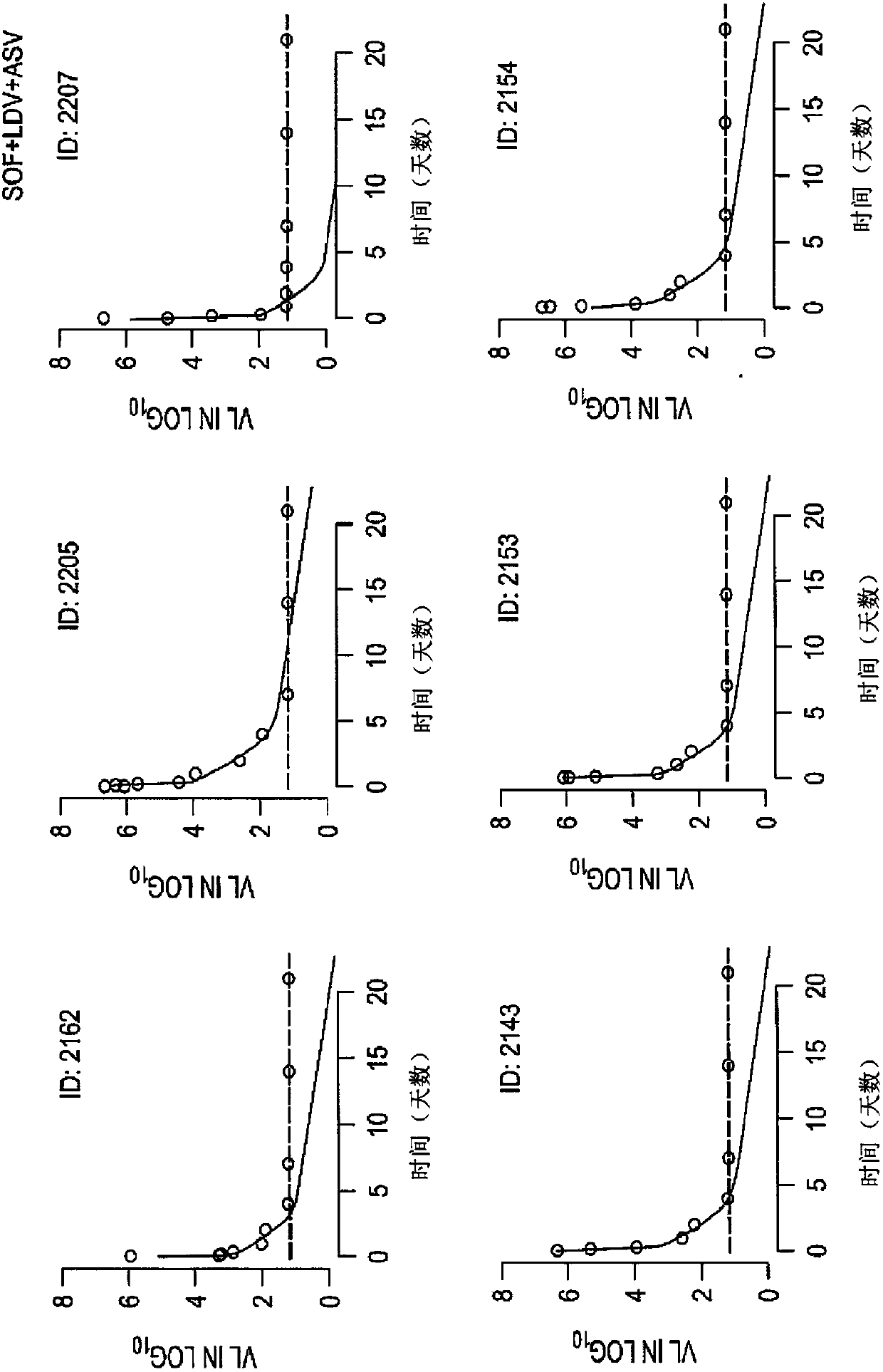
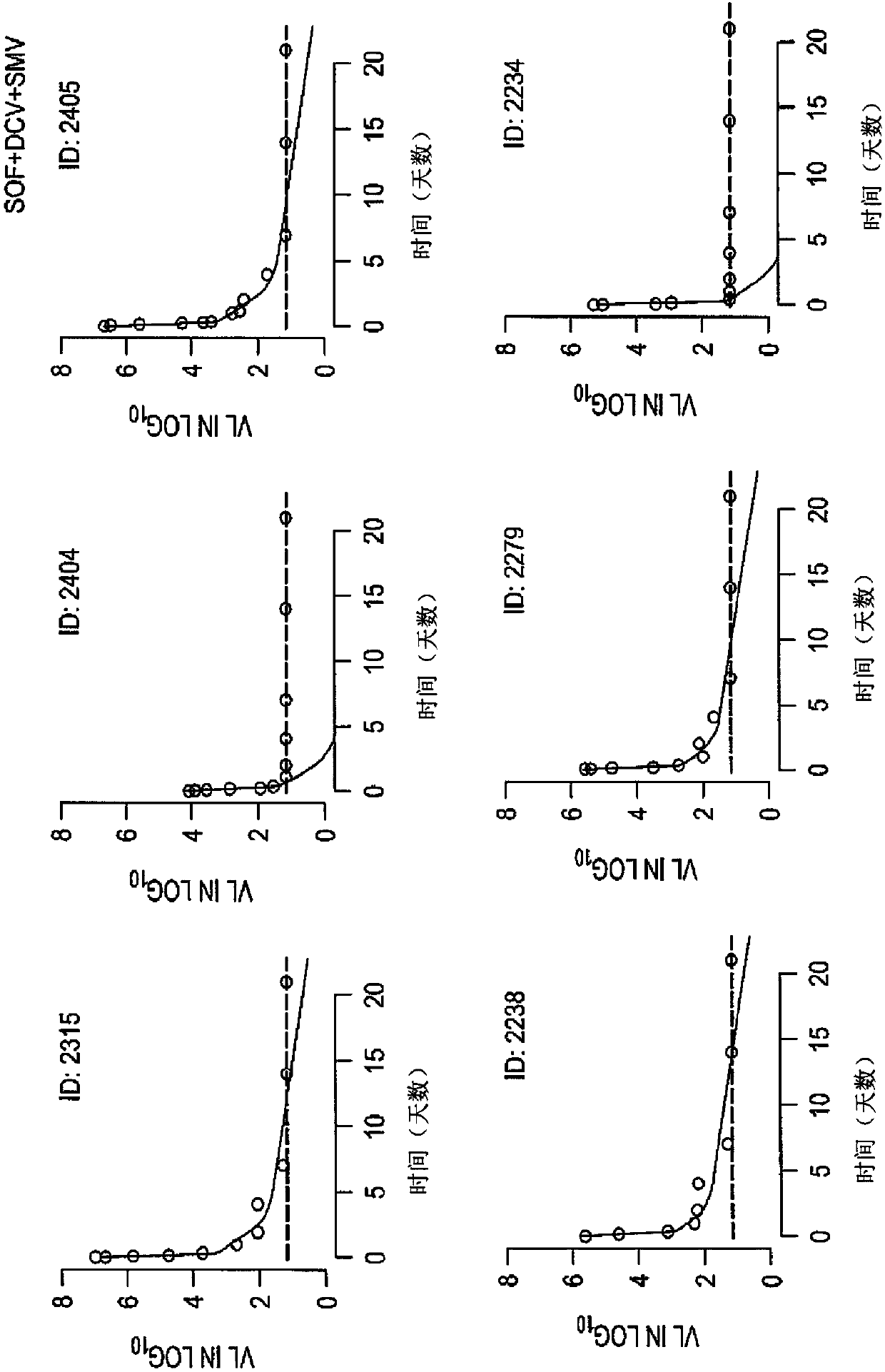
[0184] Example 1: Efficacy and Safety of a 3-Week Response-Guided Triple Direct-Acting Antiviral Therapy Against Chronic Hepatitis C Infection: A Phase 2, Open-Label, Proof-of-Concept Study
[0185] Overview:
[0186] DAA has a high cure rate and is well tolerated in patients with hepatitis C virus (HCV) infection. However, a shorter course of treatment may improve adherence, affordability, and increase DAA accessibility. We speculated that adding an NS3 protease inhibitor to the NS5A-NS5B (nucleoside) dual inhibitor could improve antiviral efficacy in individuals with rapid virological response (RVR) and shorten the duration of treatment to 3 weeks (wks), The rapid virological response (RVR) was defined as plasma HCV RNA <500 IU / ml on the second day. Therefore, the aim of this study was to examine the antiviral efficacy and safety of a 3-week response-guided therapy with an NS3 protease inhibitor and a dual NS5A inhibitor-NS5B nucleotide (HCV) infection with a course of di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com