Application of STAT3 inhibitor and STAT5 activator in enhancing lethality of NK (Natural Killer) cell to tumor cell
A technology of NK cells and tumor cells, applied in the field of immunity, can solve the problems that NK cells have no reported lethality to tumor cells, and the use of STAT5 activators has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Experimental materials
[0024] RPMI-1640 and fetal bovine serum (FBS) were purchased from GIBCO; NK cell culture medium was purchased from CellGro, Germany.
[0025] Recombinant human interleukin 2 (rhIL-2) was purchased from Shuanglu Pharmaceutical; lymphocyte separation liquid was purchased from NycomedPharma.
[0026] Human gastric cancer cells SGC-7901 and BCG-823 were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences.
[0027] 2. Experimental method
[0028] 1. Mononuclear cell isolation, grouping and NK cell culture
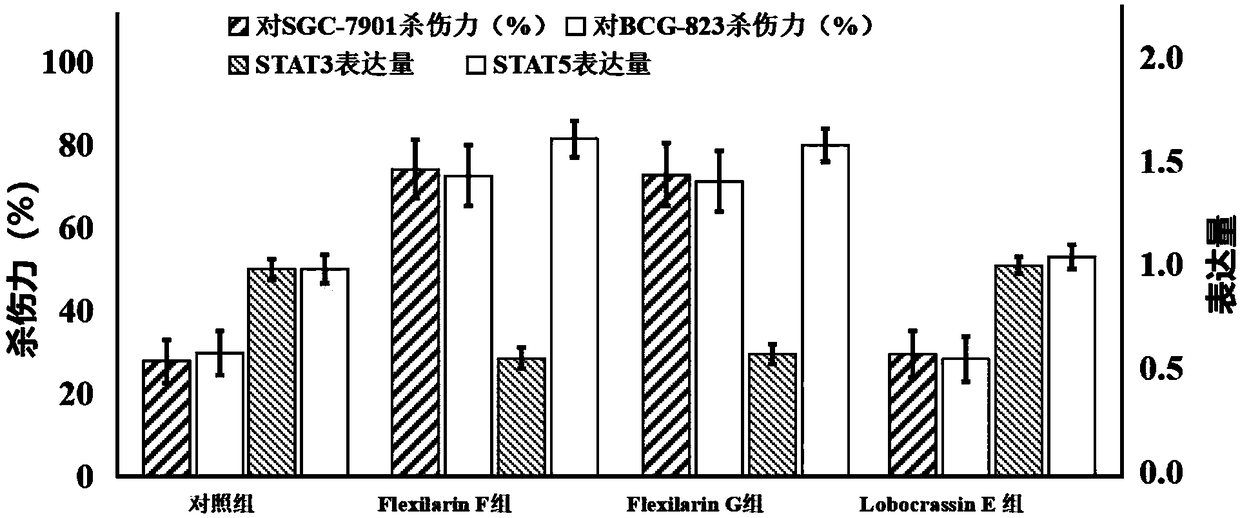
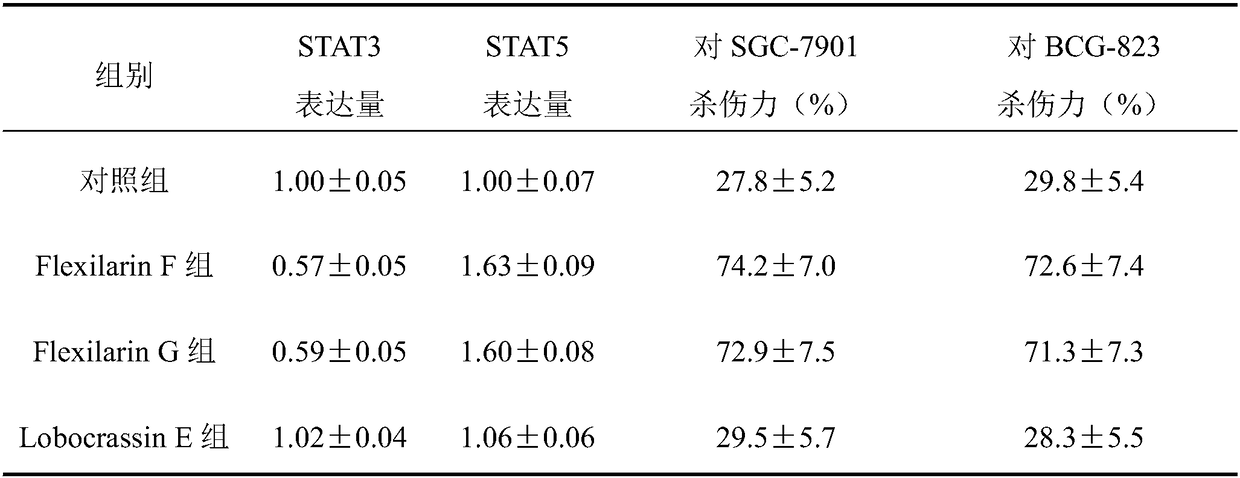
[0029] Peripheral blood from healthy volunteers was collected and subjected to Ficoll density gradient centrifugation to separate peripheral blood mononuclear cells (PBMC). On Day 0, use NK cell culture medium containing 5% autologous plasma and 80 U / ml gentamycin to adjust the PBMC to the initial cell number of 1.0×10 6 / L, divide the cells into the control group and the treatment group, the control group is routinely ...
Embodiment 2
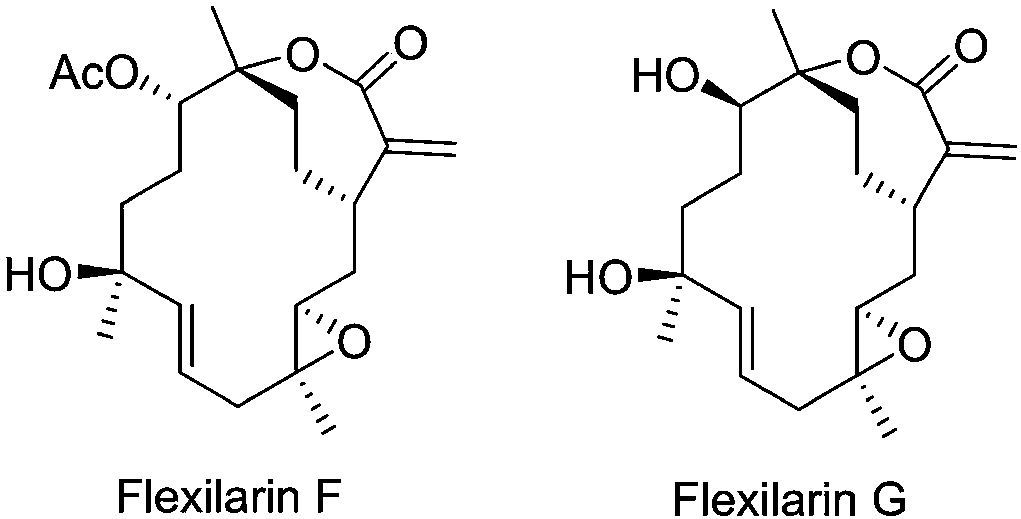
[0052] A STAT3 inhibitor, containing Flexilarin F or Flexilarin G, also contains a pharmaceutically acceptable carrier or excipient, made into a pharmaceutically acceptable dosage form; the pharmaceutically acceptable carrier or excipient includes one or more A solid, semi-solid or liquid excipient, and pharmaceutically acceptable dosage forms include tablets, capsules, granules, injections, pills, syrups, powders, and ointments.
Embodiment 3
[0054] A STAT5 activator, containing Flexilarin F or Flexilarin G, also contains a pharmaceutically acceptable carrier or excipient, made into a pharmaceutically acceptable dosage form; the pharmaceutically acceptable carrier or excipient includes one or more A solid, semi-solid or liquid excipient, and pharmaceutically acceptable dosage forms include tablets, capsules, granules, injections, pills, syrups, powders, and ointments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com