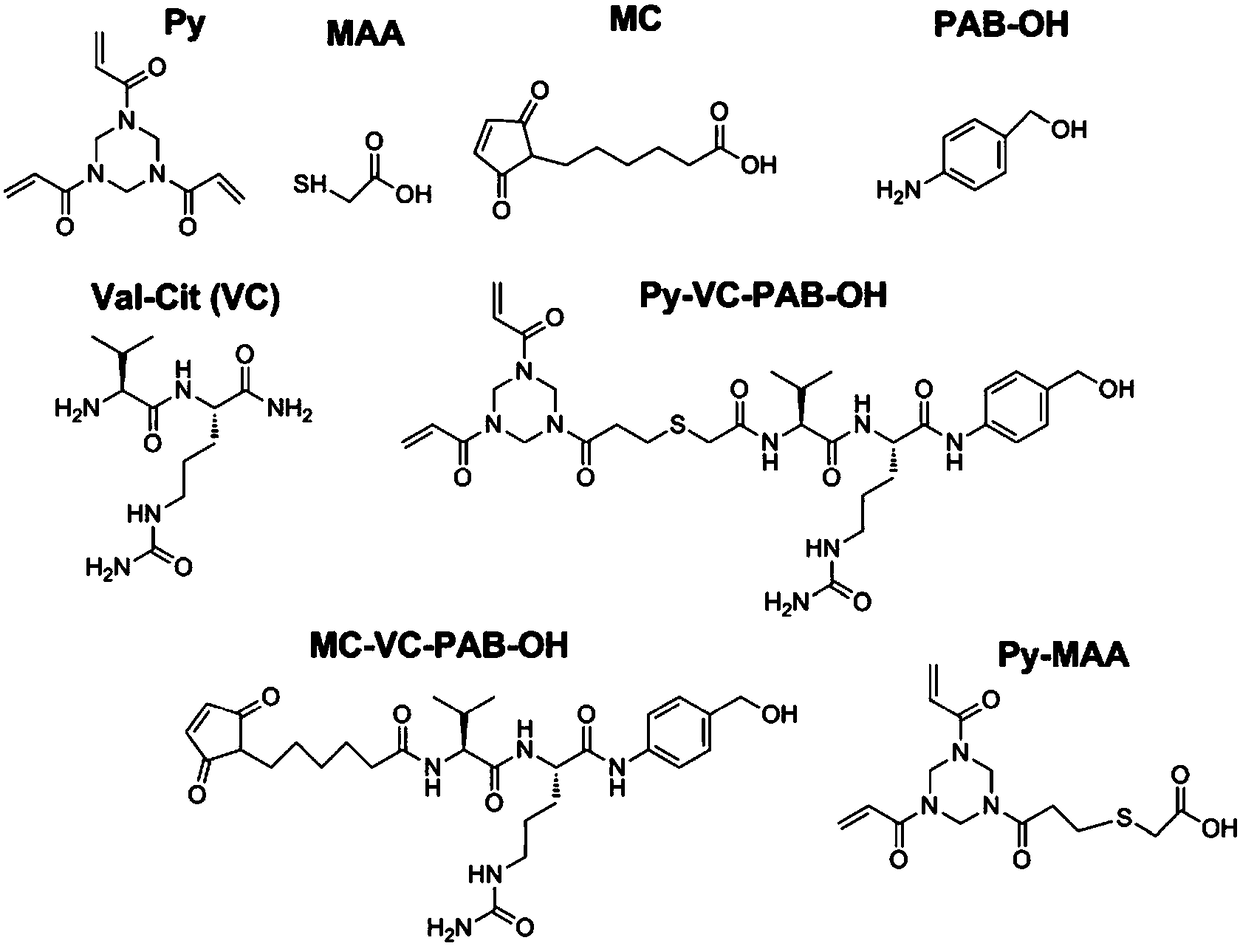
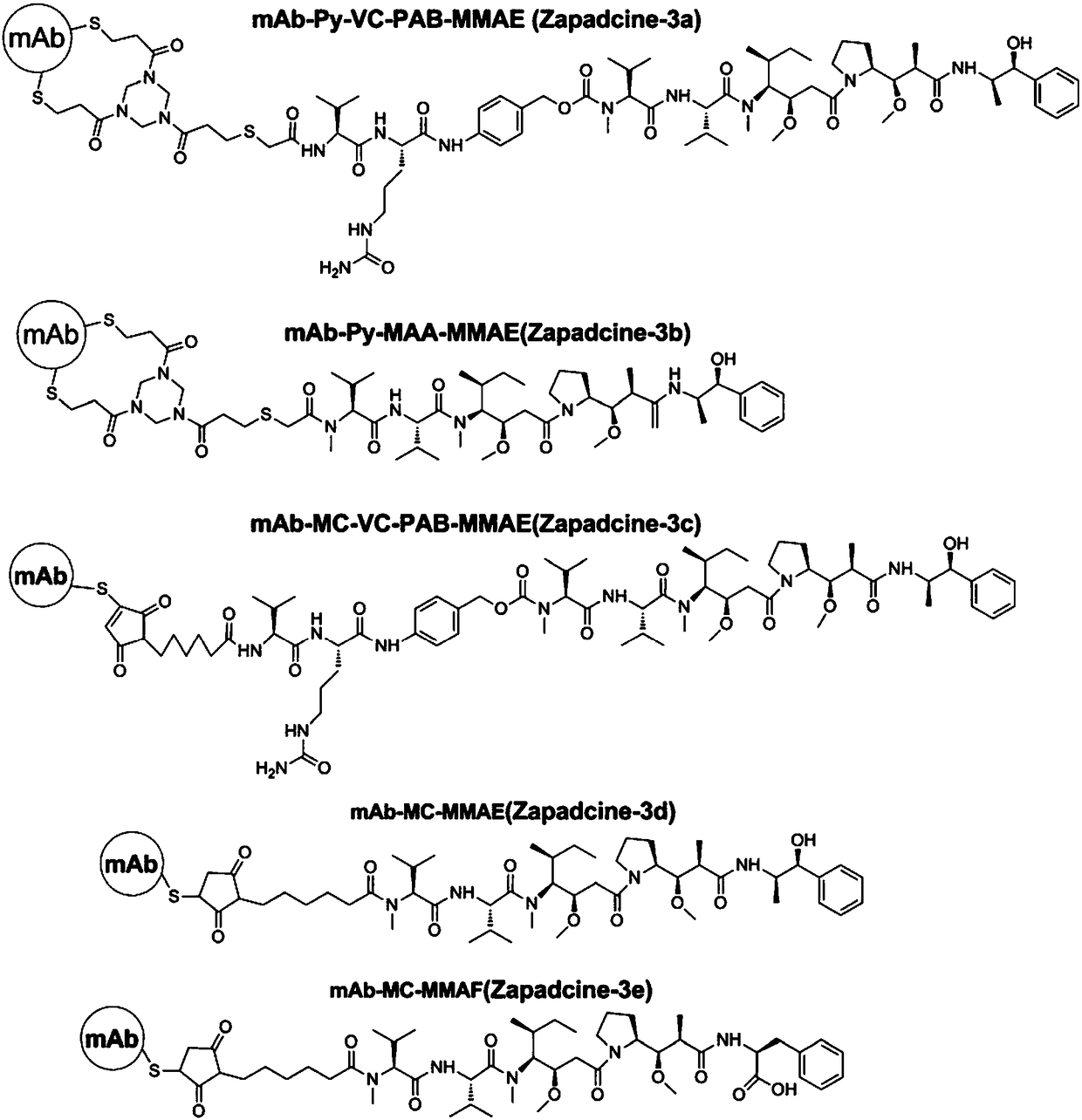
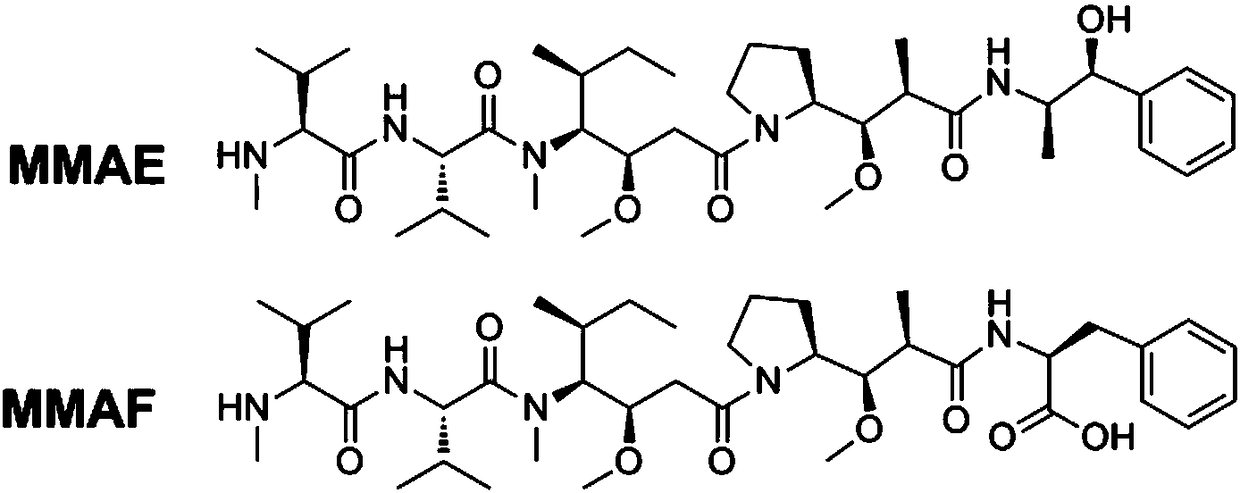
Anti-TRAILR2 antibody-toxin-conjugate and pharmaceutical application thereof in anti-tumor therapy
An antibody drug and conjugate technology, which can be used in anti-tumor drugs, drug combinations, medical preparations with inactive ingredients, etc., and can solve the problems of poor patient compliance, high cost, and high toxicity of chemical drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0198] Antibody preparation
[0199] The sequence of the DNA molecule of the antibody or fragment thereof of the present invention can be obtained by conventional techniques, such as PCR amplification or genomic library screening. In addition, the coding sequences for the light and heavy chains can be fused together to form single-chain antibodies.
[0200] Once the relevant sequences are obtained, recombinant methods can be used to obtain the relevant sequences in large quantities. Usually, it is cloned into a vector, then transformed into a cell, and then the relevant sequence is isolated from the proliferated host cell by conventional methods.
[0201]In addition, related sequences can also be synthesized by artificial synthesis, especially when the fragment length is relatively short. Often, fragments with very long sequences are obtained by synthesizing multiple small fragments and then ligating them.
[0202] At present, the DNA sequence encoding the antibody of the p...
Embodiment 1
[0269] Example 1 In vitro antitumor activity of Zapadcine-3
[0270] Various lymphocytic leukemia cell lines (Jurkat E6-1, Reh), lung cancer cell line (MSTO-211H), glioma cell line (A172), pancreatic cancer Mia PaCa-2 and human normal cells with high expression of TRAILR2 were used Lines or peripheral blood cells, such as human normal peripheral blood mononuclear cells (PBMC), human normal colonic epithelial cells (NCM-460), human normal colonic tissue cells (CCD-18Co) and human normal lung epithelial cells (BEAS-2B), To evaluate the cytotoxic effect of Zapadcine-3 on various tumor cells and normal cells. The specific research process is as follows: use trypsin (0.25%, V / V) to digest adherent cultured cells (such as MSTO-211H, etc.), detach the cells and / or directly collect suspension cultured cells (Jurkat E6-1, Reh) , resuspended in 100 μL complete medium. 5,000 adherent cells or 16,000 suspension cells were inoculated in 96-well plates for culture at 37°C overnight. Then...
Embodiment 2
[0277] Example 2 Detection of the affinity between Zapadcine-3a and TRAILR2 by ELISA
[0278] ELISA was used to evaluate the binding of Zapadcine-3a to humanized recombinant protein TRAILR2. The specific process was as follows: 2 μg / ml humanized recombinant protein TRAILR2 was coated with 1×PBS buffer (pH 7.4) at a volume of 100 μl / well 96-well plate, placed overnight at 4°C. The supernatant was discarded, and the plate was washed 3 times with PBST (PH 7.4PBS containing 0.05% Tweeen20) buffer, 5 min each time, 240 μl / well of PBS containing 5% skimmed milk powder was added, incubated at 37°C for 3 h, and blocked. Discard the blocking solution, wash the plate 3 times with 300 μl / well PBST, and add 50 μl / well of the antibody to be tested (primary antibody) or ADC gradiently diluted with PBS containing 1% skimmed milk powder or BSA, the concentration is from Start with 2-fold serial dilution at 4 μg / ml, a total of 12 concentrations, 3 replicate wells, and incubate at room tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com