Corynebacterium glutamicum capable of increasing lysine yield and constructing method of corynebacterium glutamicum
A Corynebacterium glutamicum, construction method technology, applied in the directions of microorganism-based methods, botanical equipment and methods, biochemical equipment and methods, etc., can solve NADH deficiency, limit bacterial growth sugar utilization L-lysine Acid production efficiency, insufficient content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Construction of recombinant bacterial strain C.glutamicum RGI
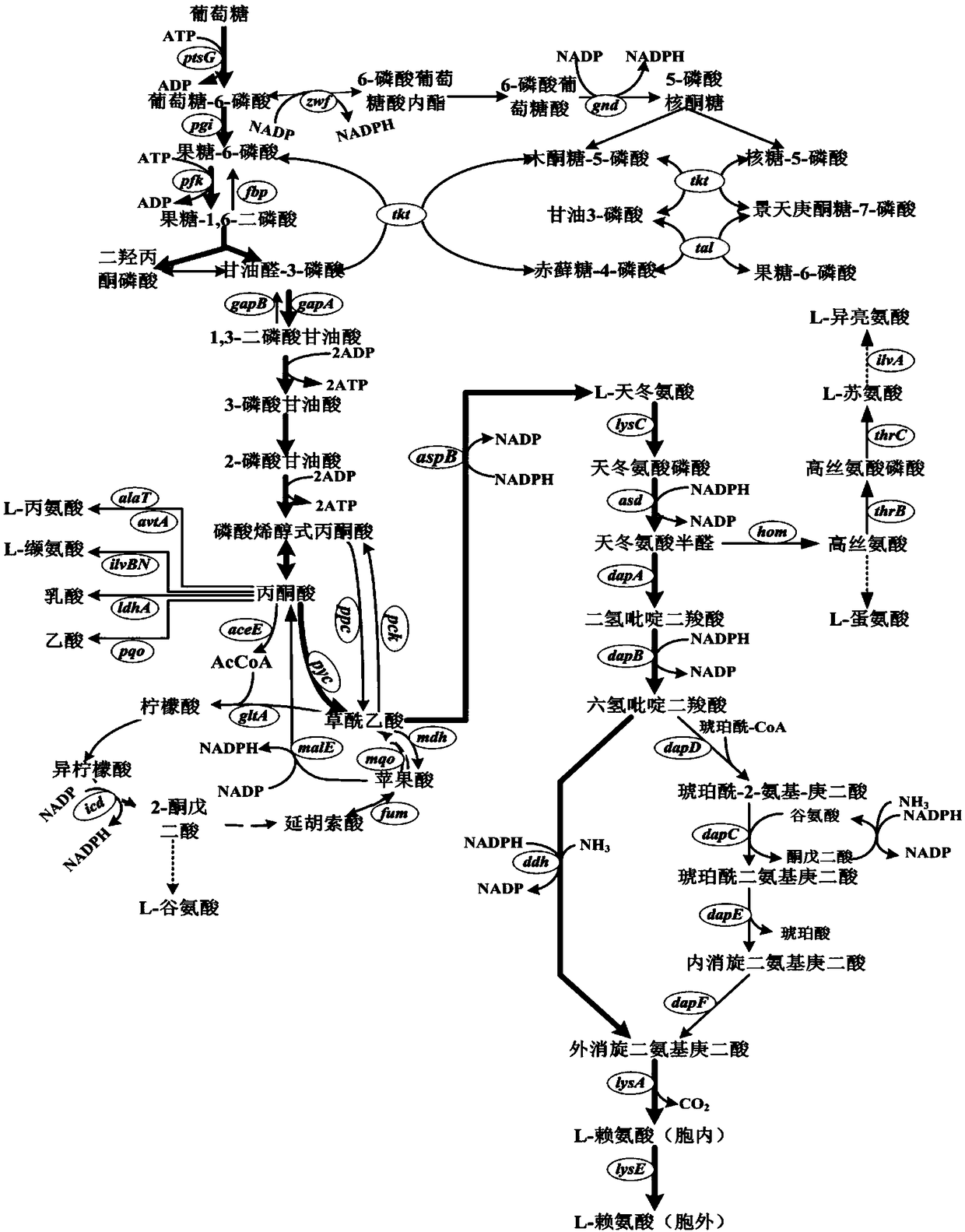
[0029] According to literature reports, NAD-IDH exists in a small number of prokaryotes such as bacteria and archaea, such as Hydrogenobacter thermophilus, A.thiooxidans, Streptococcus suis, motility fermentation Single cell bacteria (Z.mobilis) and mutans streptococcus (S.mutans). Therefore, by comparing the affinity of NAD-IDH to the cofactor NAD+ and NADP+ in the above five strains, we found that the NAD-IDH from S. + The affinity was significantly lower than that of NAD-IDH in the other four strains. Therefore, we chose NAD-IDH derived from S.mutans as a later experimental factor.
[0030] Using gene synthesis method to obtain NAD-IDH coding gene icd from S.mutans Sm , and then use the novel integrative vector pK18-MBPMT carrying antibiotic resistance and conditional lethal marker sacB double markers to replace the self-gene icd in C. glutamicum RG by two homologous recombination Cg , ...
Embodiment 2
[0039] Example 2: Determination of IDH enzyme activity of the starting bacterium C.glutamicum RG and the recombinant strain C.glutamicum RGI
[0040] Inoculate the strains preserved in the frozen tube containing 0.25g·L -1 of L-methionine and 40g·L -1 In the CgXII medium of glucose (i.e. CgXIIMG medium), shake culture at 30°C overnight, and at 10000r·min -1 Bacteria were collected by centrifugation. Subsequently, the cells were suspended in Tris-HCl buffer (pH 8.0) and subjected to ultrasonication to prepare crude enzyme solution. The crude enzyme solution was assayed by colorimetric method for enzyme activity (A 340nm ). Enzyme reaction system: 20mmol·L -1 Tris-HCl buffer (pH 8.0), 1mmol L -1 DL-trisodium isocitrate, 2mmol·L -1 MgCl 2 , 0.5mmol·L -1 NADP + or 0.5mmol·L -1 NAD + ; Reaction temperature: 30°C; Reaction time: ≥300s. One enzyme activity unit (U) is defined as the amount of enzyme required to generate 1 μmol NADPH or NADH per minute under the assay con...
Embodiment 3
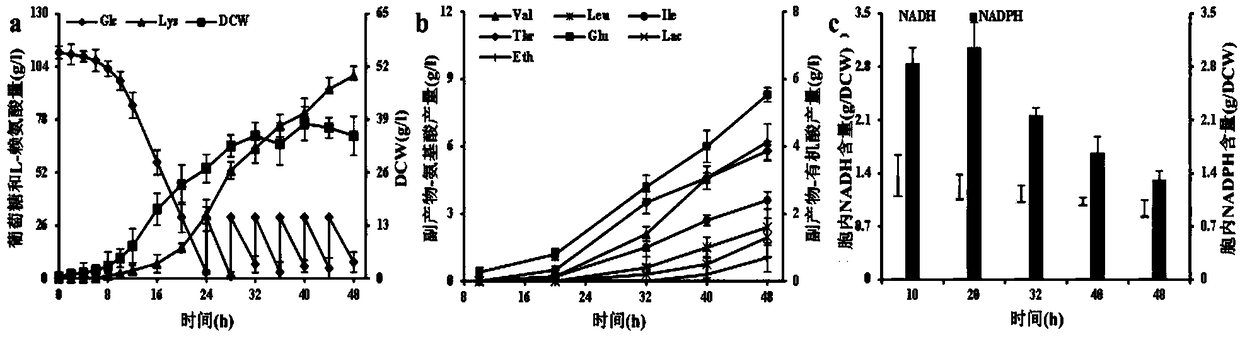
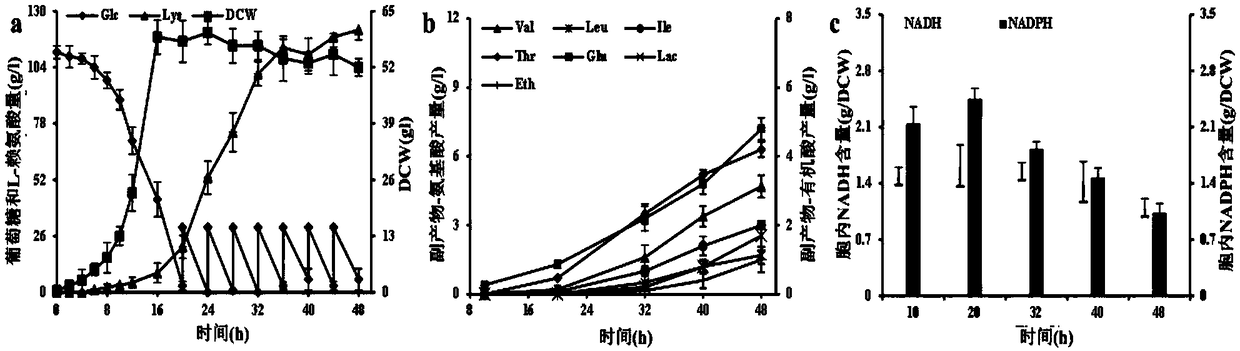
[0043] Example 3: Determination of intracellular cofactors in the starting bacterium C.glutamicum RG and the recombinant strain C.glutamicum RGI
[0044] Take a single colony and inoculate it in CgXIIMG liquid medium, 30°C, 100r min -1 Cultivate on a shaker for about 10 hours, 4°C, 6000r·min-1 The cells were collected by centrifugation, and the cells were washed three times to remove residual extracellular metabolites. Subsequently, acidic extract (0.5mol·L -1 HCl) to extract oxidized pyridine nucleotides (NAD + and NADP + ), with alkaline extract (0.5mol L - 1 NaOH) to extract reduced pyridine nucleotides (NADH and NADPH). Subsequently, with the help of the quantitative analysis kit purchased from BioVision, NAD(P) was determined by enzymatic cycle method + and NAD(P)H concentrations and calculate NADH / NAD + and NADPH / NADP + , where NAD is specifically detected with the NAD / NADH Quantification Colorimeteric Kit + and NADH, specifically detect NADP with NADP / NADPH Qua...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com