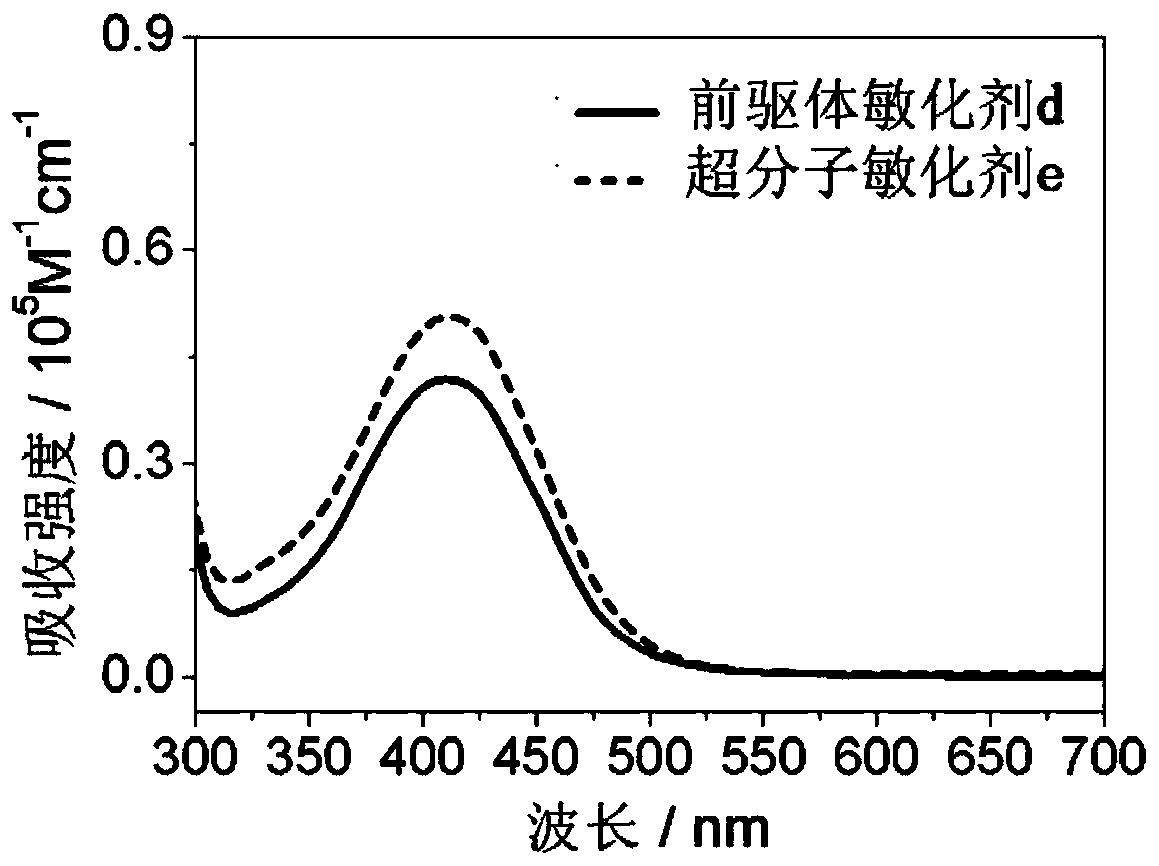
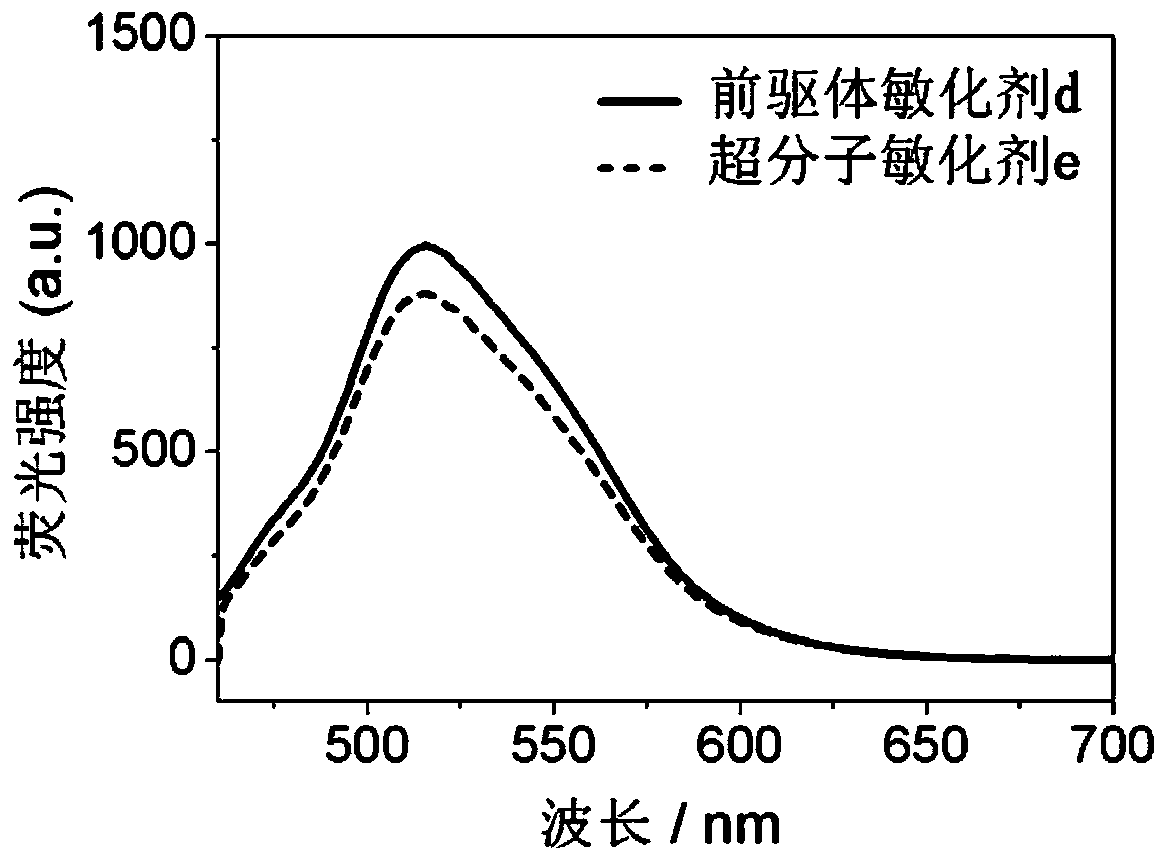
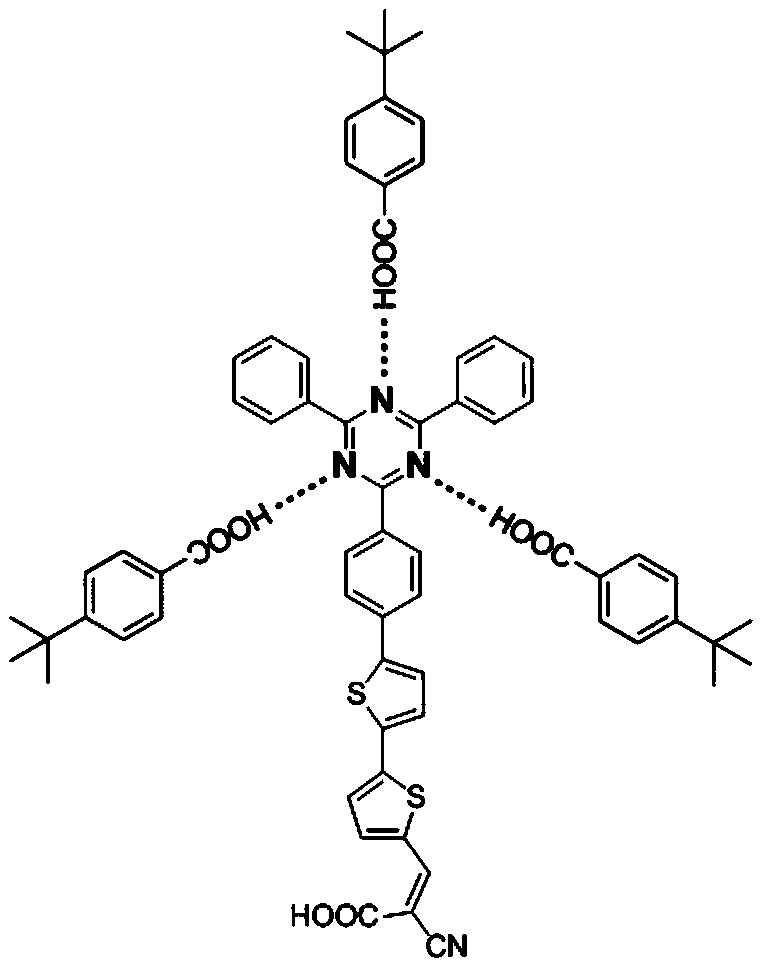
A kind of polynitrogen heterocyclic supramolecular sensitizer and its application
A sensitizer and supramolecular technology, applied in the field of sensitizers, can solve the problems of intermolecular agglomeration, affecting open circuit voltage and photoelectric conversion efficiency, etc., and achieve the effects of strong three-dimensional structure, improved photoelectric conversion efficiency, and simple molecular assembly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Prepare the polyazaheterocyclic supramolecular sensitizer e, the specific synthesis method is as follows:
[0024] 1. Preparation of compound c
[0025] Add compound a (1.0g, 2.58mmol), compound b (675mg, 2.83mmol), tetrabutylammonium bromide (250mg, 0.78mmol), bis(di-tert-butyl-4-dimethyl Aminophenylphosphine) palladium chloride (20mg, 0.03mmol), NaF (270mg, 6.44mmol) was added to a mixture of N,N-dimethylformamide (40mL) and distilled water (8mL), and reacted at 75°C for 5 hour, stop the reaction, pour the reaction solution into dichloromethane (150mL) after being cooled to room temperature, wash with water until neutral, the organic phase is dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure, column chromatography, prepared into yellow The solid compound c0.85g, the yield is 65.8%.
[0026] The nuclear magnetic data of prepared compound c is: 1 H NMR (400MHz,THF-d 8 ): δ(ppm)9.87(s,1H),8.87(d,J=8.5Hz,2H),8.84-8.81(m,4H),7.94(d,J...
Embodiment 2
[0037]The use of polynitrogen-heterocyclic supramolecular sensitizer e in Example 1 in the preparation of dye-sensitized solar cells, its specific use method and application number are 201310343718.6, and the name of the invention is "thiourea donor double bridge chain organic dye and its The method of use disclosed in the invention patent application of "Application" is the same. Simultaneously, a comparative test was performed with the compound d in Example 1. The prepared solar cells were tested using a J-V characteristic testing system (CROWNTECH IV Test Station 2000), and the results are shown in Table 1.
[0038] Table 1 Solar cell performance test results
[0039]
[0040] It can be seen from Table 1 that compared with its precursor sensitizer d, the photoelectric conversion efficiency of the solar cell prepared by using the polynitrogen heterocyclic supramolecular sensitizer e is increased by 14.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com