Cancerous human umbilical vein endothelial cell vaccine and application thereof in resisting cancers
A technology of endothelial cells and umbilical veins, applied in the direction of anti-tumor drugs, medical preparations containing active ingredients, cancer antigen components, etc., can solve problems such as limited effects, and achieve not easy to lose, long maintenance time, migration ability and invasion The effect of empowerment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0058] The tumorized HUVEC vaccine provided in this example is specifically prepared through the following steps:
[0059] (1) Collect the supernatant of CT26 cells,
[0060] In a 10cm culture dish, add 8ml of complete DMEM medium (10% BI serum + 90% DMEM) for routine culture of CT26 cells. When CT26 cells grow to occupy about 80% of the area of the culture vessel (it takes about 2 days, the cells At 80%, the cell growth belongs to the exponential growth phase, the cell viability is the strongest, and the number is the largest, which is convenient to ensure the effect of the follow-up experiment). Wash once with PBS at room temperature, clean with a vacuum pump, and then add 5ml / dish of 1640 medium containing 10% volume fraction of G16 serum (a 10cm petri dish plus 5ml of G16 medium);
[0061] After culturing for 24 hours, collect the culture medium into a 15ml or 50ml centrifuge tube, centrifuge at 3000r / min for 5min, discard the precipitate, collect the supernatant into a...
experiment example
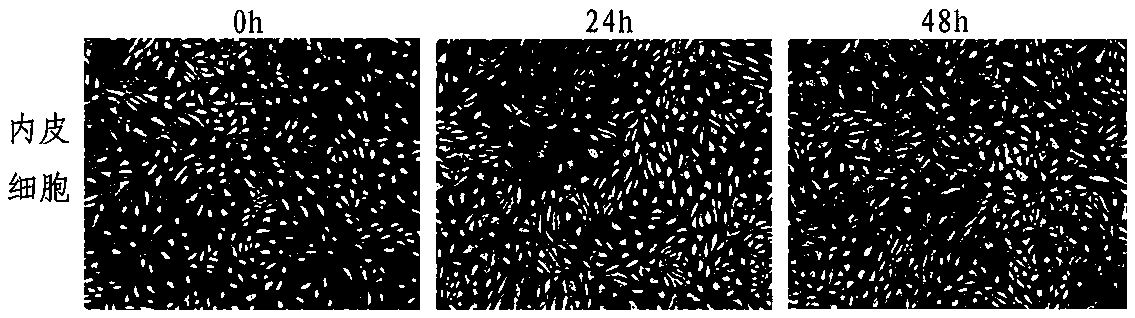
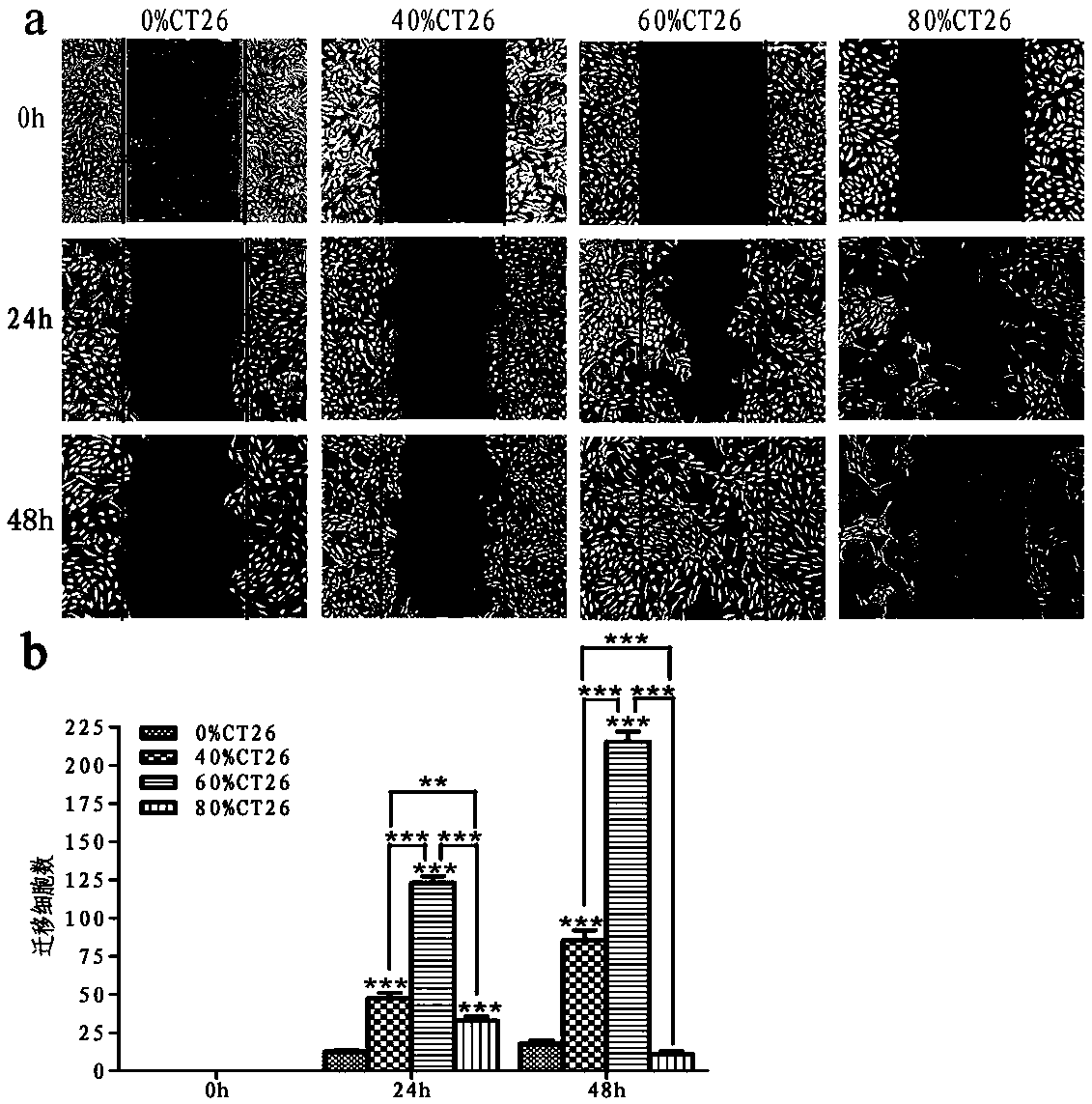
[0091] In order to test the difference in the anti-angiogenesis effect of the tumorized HUVEC vaccine prepared in the above examples and the existing HUVEC vaccine, the inventors further conducted animal experiments, which are briefly introduced as follows.
[0092] (1) Experimental grouping:
[0093] Feed female BALB / c mice (about 20-22g) aged 4-6 weeks in the barrier system, and randomly divide them into blank control group, endothelial cell group and tumorized endothelial cell group, with 7 mice in each group;
[0094] The feeding environment conditions are: temperature 20±2°C; relative humidity 50±10%; photoperiod 12h day / night; autoclaved SPF grade animal feed, litter and single distilled water; mice are free during the experiment Diet; Before the start of the experiment, the mice were pre-raised for one week to adapt to the environment;
[0095] (2) Experimental process:
[0096] After the experiment started, the corresponding vaccine was injected subcutaneously near t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com