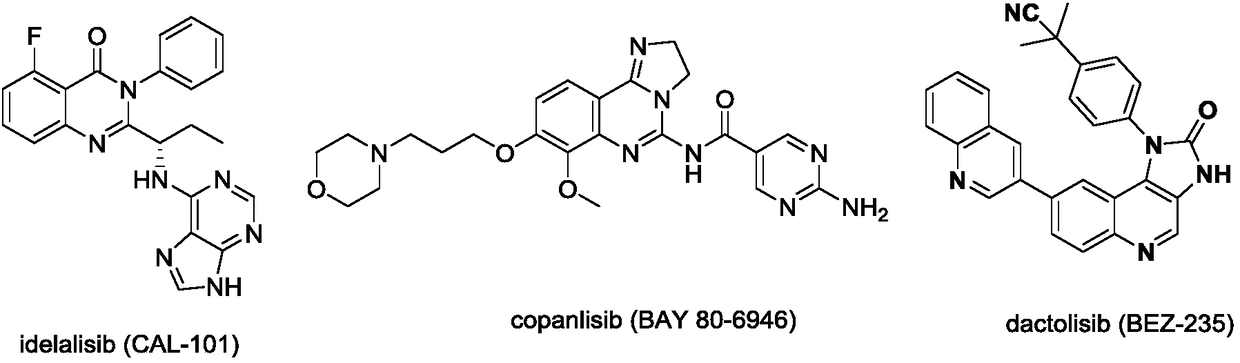
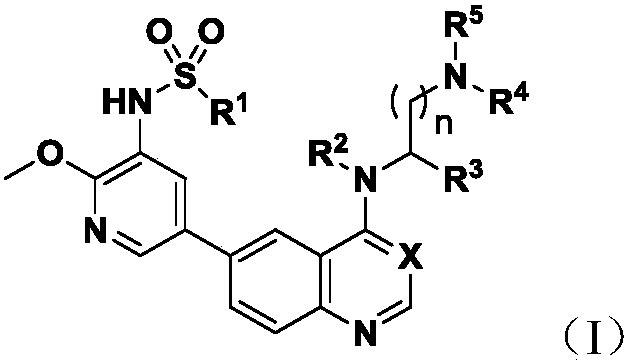
6-(Pyridine-4-yl)-4-substituted amino quinazoline or quinoline compound and application thereof
A compound, quinazoline technology, applied in organic chemistry, drug combination, bone diseases, etc., can solve the problem that the value of PI3K inhibitors has not been fully developed, and achieve good inhibitory effect and tumor volume reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
[0048] N-(2-methoxy-5-bromo-3-pyridyl)cyclopropylsulfonamide (intermediate 1-b)
[0049] Weigh 2-methoxy-3-amino-5-bromopyridine (1-a, 1.52g, 7.43mmol) into 20mL DCM, add 1.1mL pyridine, dissolve cyclopropylsulfonyl chloride (1.56g, 11.12mmol Into 10mL of DCM, add dropwise under ice bath, after dropping, react overnight at room temperature. Evaporate the solvent, extract with ethyl acetate, recrystallize to obtain 1.85g, yield 81.8%.
[0050] 4-(N-(1-piperidinylethyl))amino-6-bromoquinazoline (intermediate 1-d)
[0051] Weigh 4-chloro-6-bromoquinazoline (1-c, 2.01g, 8.01mmol) in isopropanol (15mL), add 1-(2-aminoethyl) piperidine (1.05g, 8.01mmol ), heated to reflux for 5 h under the protection of nitrogen, cooled to room temperature, and filtered to obtain white solid 1-d (2.74 g, 74.6%). mp:215.0-217.5℃. 1 H NMR (CDCl 3 )δ8.84(s,1H,NH),8.78(d,J=2.0Hz,1H,Ar-H),8.60(s,1H,Ar-H),7.81(dd,J=8.9,2.1Hz, 1H, Ar-H), 7.68(d, J=8.9Hz, 1H, Ar-H), 4.22-4.12(t, 2H, CH 2 )...
Embodiment 2
[0055]
[0056] 4-(N-(4-morpholinoethyl))amino-6-bromoquinazoline (intermediate 2-b)
[0057] The same preparation method as intermediate 1-d, white solid (2.07g, 72.1%). mp:204.0-205.6℃. 1 H NMR (CDCl 3 ) δ8.65(s,1H,Ar-H),8.27(s,1H,Ar-H),7.84(dd,J=8.9,2.0Hz,1H,Ar-H),7.73(d,J=8.9 Hz, 1H,Ar-H),4.00(s,4H,CH 2 ),3.94(s,2H,CH 2 ),3.07(s,2H,CH 2 ),2.93(s,4H,CH 2 ).ESI-MS m / z:338.1 / 340.1[M+H] + .
[0058] N 1 -(2-Methoxy-5-((4-(N 2 -(4-morpholine ethyl))amino)-6-quinazoline)-3-pyridyl)cyclopropylsulfonamide (compound 2)
[0059] The preparation method was the same as that of compound 1 to obtain compound 2 (80.2 mg, 55.7%). mp:64.2-66.8℃. 1 H NMR (DMSO-d 6 ) δ8.54(s,1H,NH),8.50–8.44(m,2H,Ar-H),8.42(s,1H,Ar-H),8.07(d,J=8.5Hz,2H,Ar-H ), 7.76(d, J=8.6Hz, 1H, Ar-H), 4.00(s, 3H, OCH 3 ), 3.69 (d, J=6.0Hz, 2H, CH 2 ),2.80–2.70(m, 1H,CH), 2.57(t,J=6.9Hz,2H,CH 2 ),2.43(s,4H,CH 2 ×2),1.49(d,J=4.9Hz,4H,CH 2 ×2), 1.38(d,J=4.2Hz,2H,CH 2 ),0.95(d,J=7.4Hz,2H,CH 2 ).ESI-MS ...
Embodiment 3
[0061]
[0062] N-(2-methoxy-5-bromo-3-pyridyl)methylsulfonamide (intermediate 3-b)
[0063] Weigh 2-methoxy-3-amino-5-bromopyridine (1-a, 0.94g, 4.63mmol) into 20mL DCM, add 1.1mL pyridine, dissolve methanesulfonyl chloride (0.43mL, 5.56mmol in In 10mL of DCM, add dropwise under ice-cooling, after dropping, react overnight at room temperature. Evaporate the solvent, extract with ethyl acetate, recrystallize to obtain 1.05g, yield 80.8%.
[0064] N 1 -(2-Methoxy-5-((4-(N 2 -(1-piperidinyl))amino)-6-quinazoline)-3-pyridyl)methylsulfonamide (compound 3)
[0065] The preparation method was the same as that of compound 1 to obtain compound 3 (58.6 mg, 55.7%). mp:120.6-123.5℃. 1 H NMR (DMSO-d 6 )δ8.53(d,J=1.5Hz,1H,NH),8.50–8.38(m,3H,Ar-H),8.11–8.01(m,2H,Ar-H),7.76(d,J=8.7 Hz,1H,Ar-H),3.99(s,3H,OCH 3 ), 3.69 (dd, J=12.9, 6.4Hz, 2H, CH 2 ),3.08(s,3H,CH 3 ), 2.58(t, J=7.0Hz, 2H, CH 2 ),2.44(s,4H,CH 2 ×2), 1.50(dt, J=10.7, 5.5Hz, 4H, CH 2 ×2), 1.38(d, J=4.8Hz, 2H, CH 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com