A kind of recovery method of avibactam intermediate isomer
A recovery method and isomer technology, which is applied in the field of drug synthesis and recovery treatment, can solve the problems of unprotected NH groups, affecting the purity and quality of products, and strong oxidizing properties of oxidants, achieving mild reaction conditions, efficient recovery, and environmental protection. The effect of less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
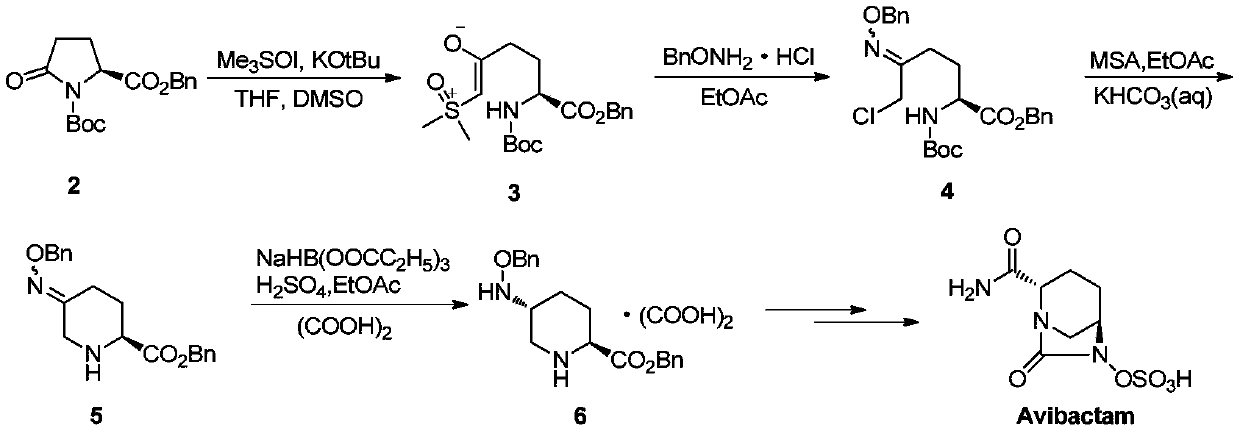
[0033] This example is for the recovery of formula II compound (2S,5S)-5-((benzyloxy)amino)piperidine-2-carboxylate intermediate from the mother liquor produced during the salt-forming process of the avibactam intermediate Isomers, wherein R in the compound of formula II is benzyl;
[0034]
[0035] The specific recycling process is as follows:
[0036] Take 18 g of the starting material (S)-N-tert-butyl formate-5-oxopyrrolidine-2-carboxylate, carry out the synthesis operation according to the example 1a method disclosed in the international patent application WO2012172368, and filter to obtain (2S, 5R)-5-((benzyloxy)amino)piperidine-2-carboxylic acid benzyl oxalate, collect about 100mL of the filtrate (mother liquor) after the salt-forming reaction, and then add 50mL of 5wt% sodium carbonate to the filtrate Stir and wash the aqueous solution once, adjust the pH value to 7-8, wash twice with 50mL of water, combine the organic phases, dry with anhydrous sodium sulfate, filt...
Embodiment 2
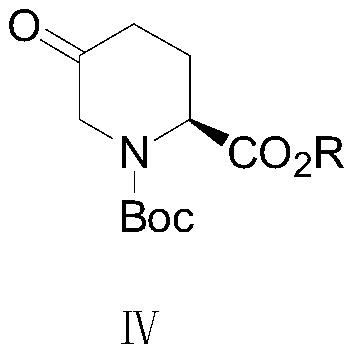
[0039] In this embodiment, the specific preparation process of the corresponding compound of formula III can be obtained by the following method, wherein the R group in the compound of formula IV corresponds to the benzyl group of the R group in the compound of formula II;
[0040]
[0041] Take 7 g of the recovered formula II compound (2S,5S)-5-((benzyloxy)amino)piperidine-2-carboxylate 7 g, add 40 mL of acetonitrile solvent and 2.3 g of triethylamine, and then add amino Protecting group Boc 2 O (di-tert-butyl dicarbonate) 4.9g, control the temperature and react at room temperature for 4 hours, after the reaction, remove the solvent by rotary evaporation, then add 80mL of ethyl acetate to dilute, and wash the organic phase with 50mL of 1% dilute hydrochloric acid Twice, stand still, separate the liquid, collect the organic phase and wash it once with 50 mL of water, dry the collected organic phase with anhydrous sodium sulfate, filter, and concentrate the filtrate to remov...
Embodiment 3
[0043] In this embodiment, the specific preparation process of the corresponding compound of formula I can be obtained by the following method, wherein R in the compound of formula I is benzyl.
[0044]
[0045] Take 4.7g of the ketone intermediate represented by the compound of formula IV and put it into the reaction vessel, add 2.4g of benzyloxyamine hydrochloride, 2.3g of sodium acetate and 30mL of methanol solvent, then slowly raise the temperature and control the temperature at 60°C Condensation reaction was carried out for 3 hours, after the condensation reaction was completed, hydrogen chloride gas was introduced to carry out the deprotection reaction until the reaction was complete, the methanol solvent was evaporated under reduced pressure, 50 mL each of ethyl acetate and water were added to the residue, and stirred for 0.5 hours, Static extraction, liquid separation, the collected organic phase was washed twice with 30mL saturated sodium bicarbonate, then dried wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com