Cattle-sourced hyaluronidase affinity medium and adsorption method thereof
A technology of hyaluronidase and hyaluronic acid, applied in chemical instruments and methods, separation methods, solid adsorbent liquid separation, etc., can solve problems such as inability to carry out industrial production, low recovery rate, and complicated operation, and achieve large-scale industrial production Application potential, great economic benefit, effect of increased purification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
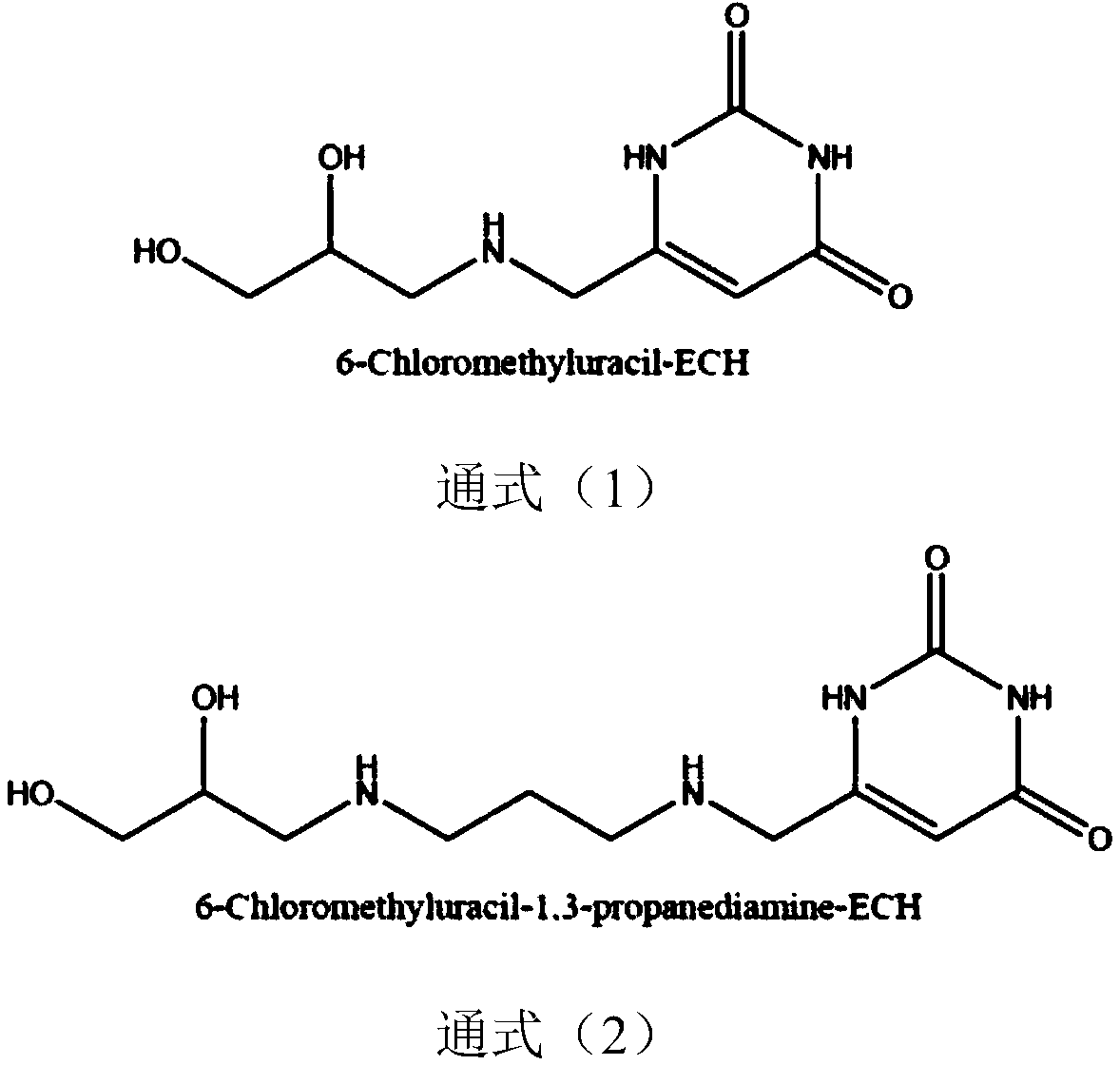
[0029] Embodiment 1: Synthesis and application of Sepharose-6-Chloromethyluracil-ECH
[0030] Sepharose CL 4B (100g) was washed with 10 times the volume of deionized water, and dried into a wet cake; suspended in 50ml activation buffer (0.8M NaOH, 20% DMSO, 10% Epichlorohydrin, 0.5mg / ml sodium borohydride) Shake at 40°C for 2.5 hours, then pour into a glass frosted funnel, and wash with 10 times the volume of distilled water each time under suction filtration until the pH of the washing solution is neutral, and then drained to form a wet cake. Suspend the activated Sepharose CL 4B medium in 500ml 0.1M NaOH solution, add 20mL ammonia water, keep the gel at 30°C for 12h under stirring (200rpm), and wash it with deionized water. Weigh 2g of 6-Chloromethyluracil, mix with 20g of the washed medium at 60°C for 12h, maintain pH 8.0, and wash with deionized water. The medium Sepharose-6-Chloromethyluracil-ECH is obtained, the structure of which is shown in the general formula (1), re...
Embodiment 2
[0039] Example 2: Synthesis and application of Sepharose-6-Chloromethyluracil-1,3-propanediamine-ECH
[0040]Sepharose CL 4B (100g) was washed with 10 times the volume of deionized water, and dried into a wet cake; suspended in 50ml activation buffer (0.8M NaOH, 20% DMSO, 10% Epichlorohydrin, 0.5mg / ml sodium borohydride) Shake at 40°C for 2.5 hours, then pour into a glass frosted funnel, and wash with 10 times the volume of distilled water each time under suction filtration until the pH of the washing solution is neutral, and then drained to form a wet cake. Suspend the activated Sepharose CL 4B medium in 500ml 0.1M NaOH solution, add 20ml 1,3-propanediamine, keep the gel at 30°C for 12h under stirring (200rpm), and wash it with deionized water. Weigh 2g of 6-Chloromethyluracil, mix with 20g of the washed medium at 60°C for 12h, maintain pH 8.0, and wash with deionized water.
[0041] The medium Sepharose-6-Chloromethyluracil-1,3-propanediamine-ECH is obtained, the structure ...
Embodiment 3
[0048] Embodiment 3: Synthesis and application of Sepharose-2-Aminobenzonzimidazole-ECH
[0049] Sepharose CL 4B (100g) was washed with 10 times the volume of deionized water, and dried into a wet cake; suspended in 50ml activation buffer (0.8M NaOH, 20% DMSO, 10% Epichlorohydrin, 0.5mg / ml sodium borohydride) Shake at 40°C for 2.5 hours, then pour into a glass frosted funnel, and wash with 10 times the volume of distilled water each time under suction filtration until the pH of the washing solution is neutral, and then drained to form a wet cake. Suspend 20g of activated Sepharose CL 4B medium in 50ml of 0.1M NaOH solution, weigh and add 2g of 2-Aminobenzimidazole, keep the temperature at 60°C for 12h, and wash with deionized water.
[0050] The medium Sepharose-6-Chloromethyluracil-ECH was obtained, the structure of which was shown in the general formula (3), referred to as structure 3.
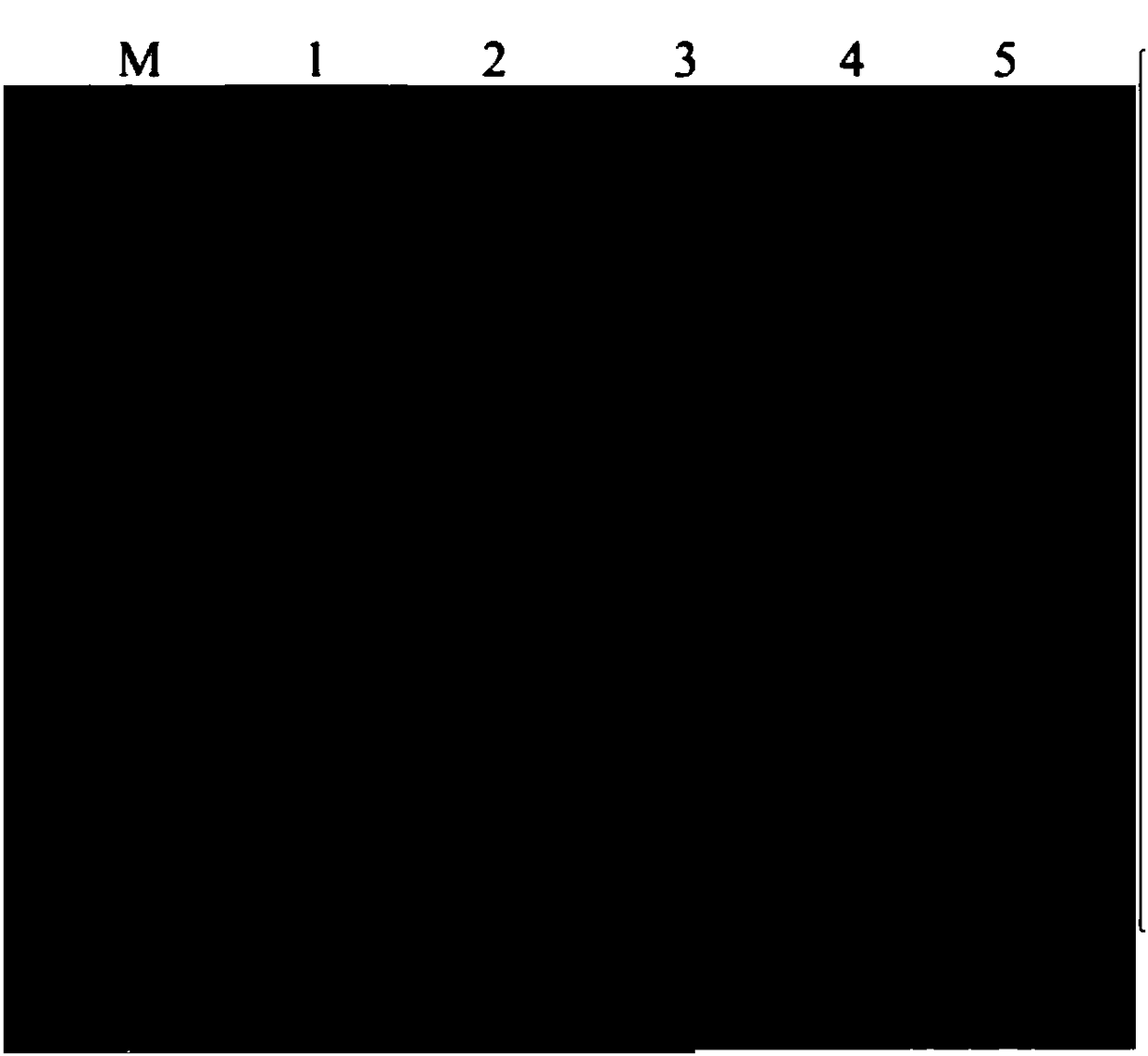
[0051] (1) Determination of the maximum adsorption capacity of the medium
[0052] Det...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com