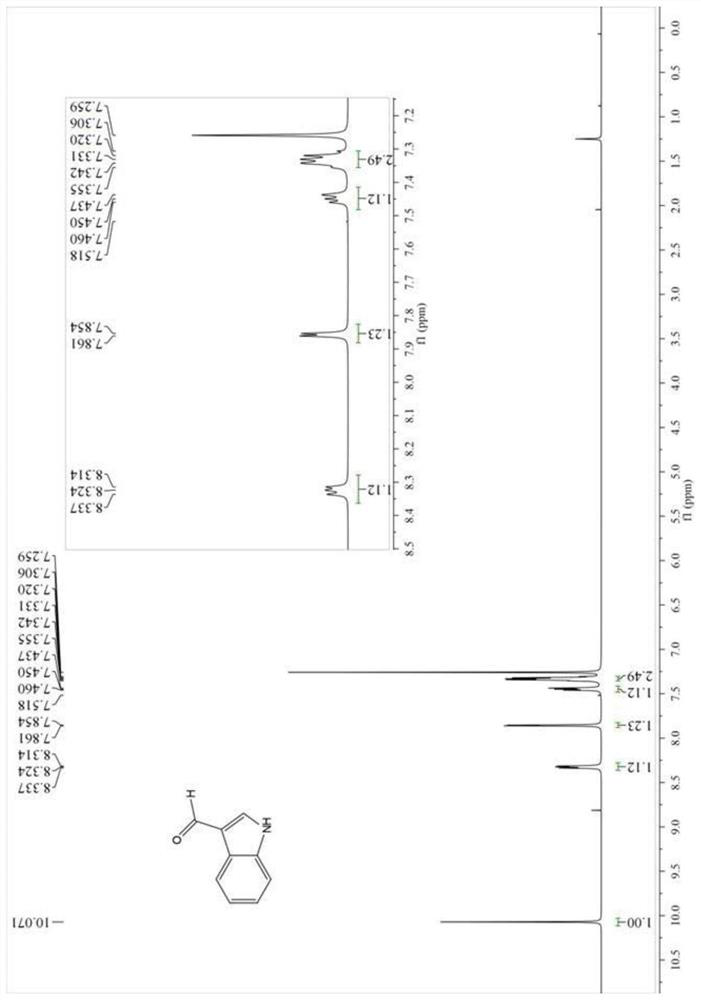
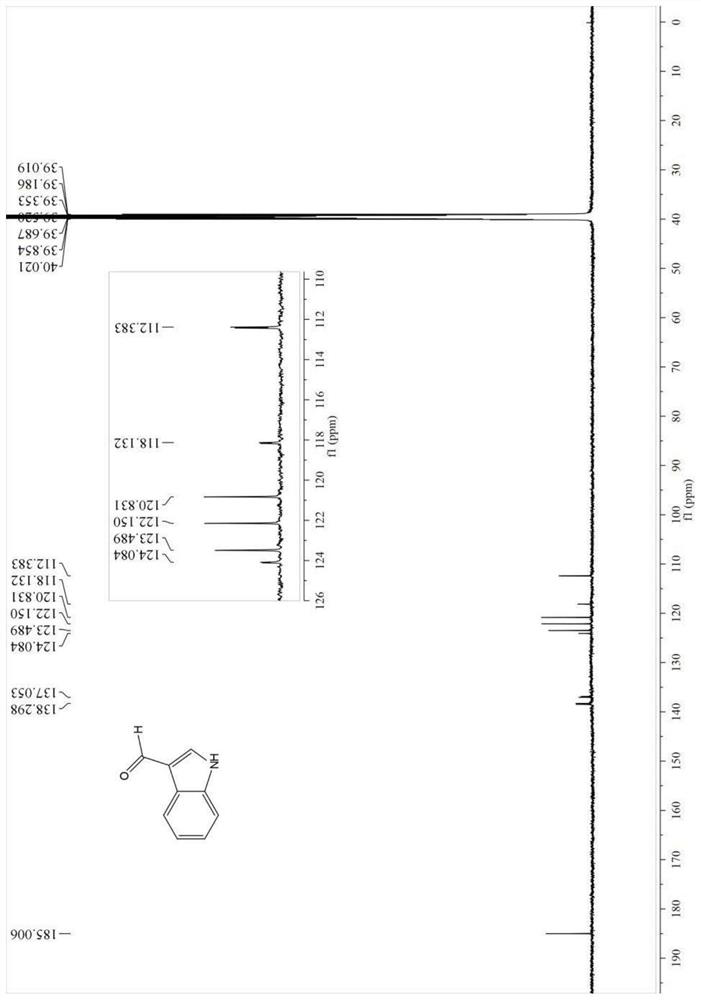
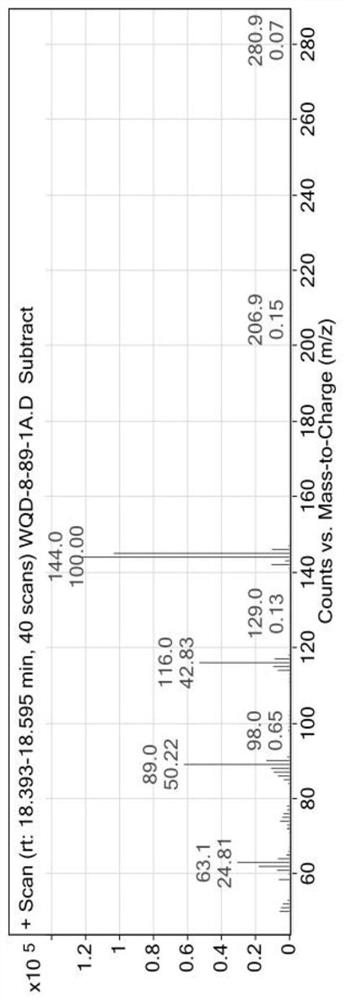
A method for synthesizing indole-3-carboxaldehyde compounds
A compound and formaldehyde technology, applied in the field of synthesizing indole-3-carboxaldehyde compounds, can solve the problems of unfavorable large-scale production, high cost of raw materials, complicated and difficult to control post-treatment process, etc., and achieve broad group compatibility, reaction Effect of low conditional requirements and broad substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] R in the general formula of compound III 1 , R 2 , R 3 Simultaneously is the synthetic method of the compound III-1 of hydrogen, described method comprises the following steps:
[0043] (1) Add 1.0 mmol indole (R in the general formula of compound I) to a 50 mL round bottom flask 1 , R 2 , R 3 At the same time hydrogen) and 1.0mmol (0.140g) hexamethylenetetramine, then add 2mL N,N-dimethylformamide (DMF), put in a magnetic stir bar to stir to dissolve the solid, then add 0.05mmol (0.012g ) crystallized aluminum trichloride, connected to a reflux condenser and heated at 120°C, monitored the reaction process with TLC, cooled to room temperature after reacting for 1h, and obtained a suspension;
[0044] (2) The suspension obtained in step (1) is suction-filtered with a funnel lined with diatomaceous earth, the filter cake is fully washed with ethyl acetate, suction-filtered, and the above operations are repeated until the filtrate has no product, and all the filtrates...
Embodiment 2
[0047] R in the general formula of compound III 1 , R 3 Also for hydrogen, R 2 Synthesis of Compound III-2 as Phenyl.
[0048] Compound III-2 is prepared in the same manner as in Example 1. The difference between this example and Example 1 is: (1) the compound I used in this example is 2-phenylindole (R in compound I general formula 1 , R 3 Also for hydrogen, R 2 is phenyl); (2) the reaction time in step (1) is 2h.
[0049] The quality of the target product obtained in this example was 0.171 g, and the yield was 77%.
[0050] The test results of the target product 2-phenylindole-3-carbaldehyde synthesized in this example are as follows: m.p.250-252°C. 1 H NMR (400MHz, DMSO-d 6 )δ12.41(s,1H),9.97(s,1H),8.22(d,J=7.6Hz,1H),7.83–7.73(m,2H),7.66–7.54(m,3H),7.51(d ,J=7.8Hz,1H),7.33–7.21(m,2H). 13 C NMR (101MHz, DMSO-d 6 )δ185.49, 149.07, 135.89, 129.88, 129.83, 129.77, 128.97, 125.75, 123.70, 122.43, 121.05, 113.46, 112.00. EI-MS m / z (%) 89(12), 139(9), 165(43) ,191(15),2...
Embodiment 3
[0052] R in the general formula of compound III 1 , R 2 Also for hydrogen, R 3 =4-CH 3 Synthesis of Compound III-3.
[0053] Compound III-3 is prepared in the same manner as in Example 1. The difference between this example and Example 1 is: (1) the compound I used in this example is 4-methylindole (R in the general formula of compound I) 1 , R 2 Also for hydrogen, R 3 =4-CH 3 ); (2) the reaction time in step (1) is 2.4h.
[0054] The quality of the target product obtained in this example was 0.123 g, and the yield was 78%.
[0055] The test results of the target product 4-methylindole-3-carbaldehyde synthesized in this example are as follows: m.p.189-191°C. 1 H NMR (400MHz, DMSO-d 6 )δ12.20(s,1H),9.91(s,1H),8.24(d,J=2.9Hz,1H),7.31(d,J=8.1Hz,1H),7.13(t,J=7.7Hz, 1H), 6.98(d, J=7.2Hz, 1H), 2.77(s, 3H). 13 C NMR (101MHz, DMSO-d 6 )δ184.16,139.06,137.87,131.18,123.53,123.37,123.34,119.79,109.99,22.26.EI-MS m / z(%)51(8),77(23),103(16),130(47), 158(100),159(85)(M + ). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com