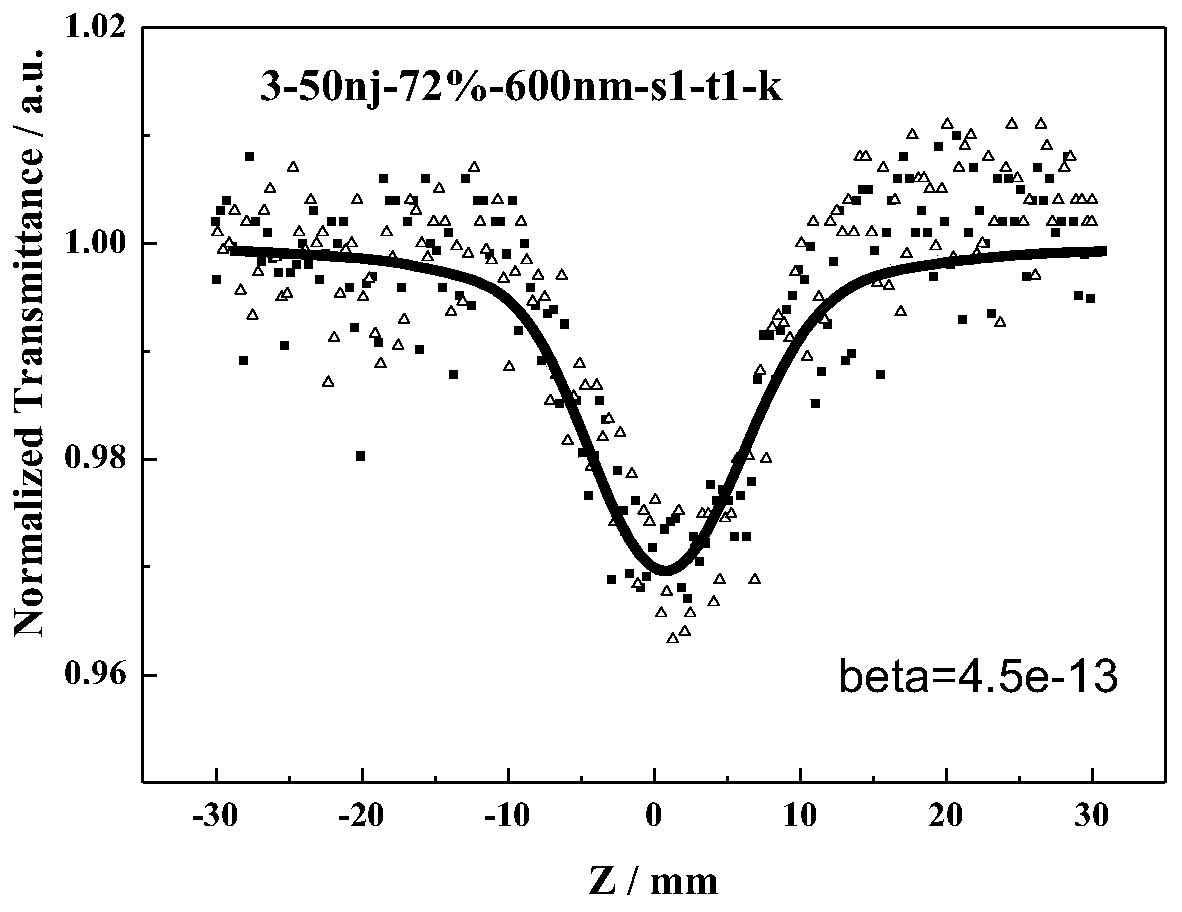
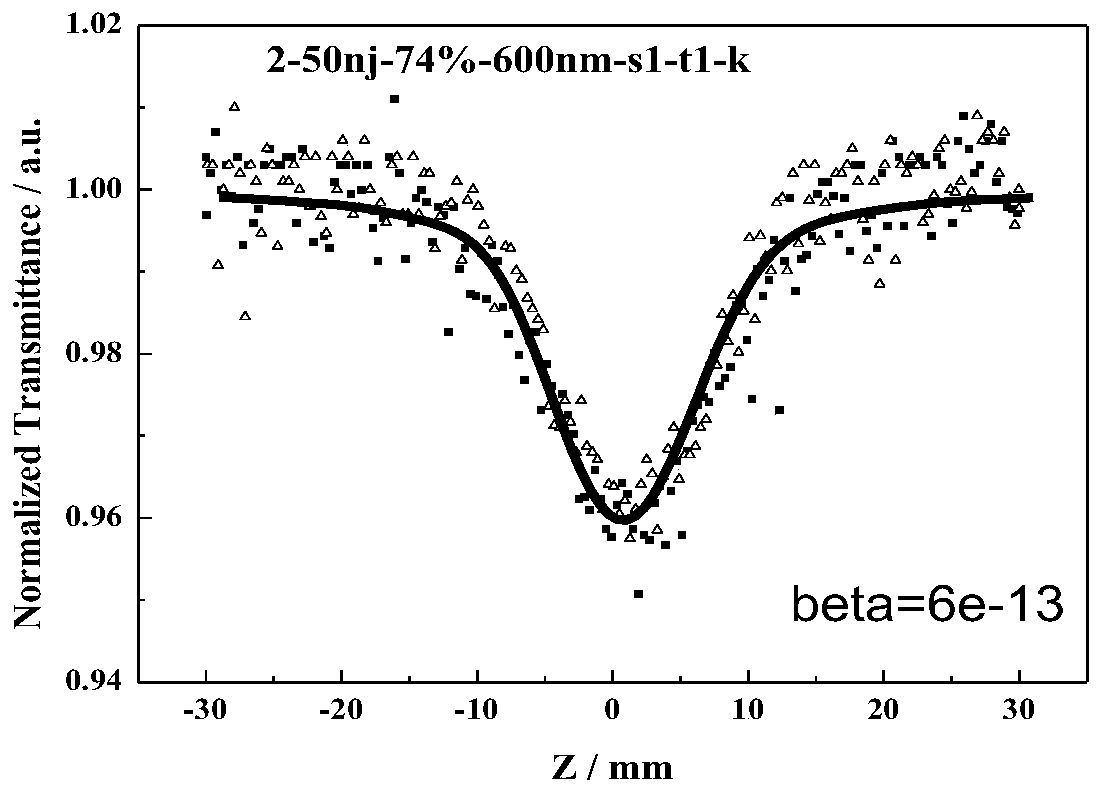
Azo compound with nonlinear optical properties and its synthesis method
An azo compound and nonlinear optics technology, applied in the field of new azo compounds and their synthesis, can solve the problems of less research on azo compound materials, small molecular weight of azo compounds, inhibition of nonlinear characteristics, etc. The effect of single product, easy operation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Synthesis of 5-(1-(2'-amino-5'-sodium sulfonate phenyl)diazenyl)-1-naphthalenesulfonate sodium azo compound
[0037] At room temperature, weigh sodium nitrite (0.6900g, 0.01mol), 5-amino-1-naphthalenesulfonic acid (2.2325g, 0.01mol) and anhydrous sodium carbonate (1.6958g, 0.016mol) and dissolve in 100ml distilled water , and stir to make it evenly mixed to form a mixed liquid A.
[0038] Dilute concentrated hydrochloric acid (1.75ml, 0.02mol) into 1mol / L dilute hydrochloric acid. Under ice-water bath conditions, the mixed solution A was added dropwise in dilute hydrochloric acid, and solution B was formed after ten minutes of reaction; under ice-water bath conditions, p-aminobenzenesulfonic acid (1.7319g, 0.01mol), sodium hydroxide (0.8 g, 0.02mol) was dissolved in 40ml of distilled water to form solution C.
[0039]Under the condition of ice-water bath, solution B was added dropwise to solution C, and the reaction was continued for 3-5 hours;
[0040] Af...
Embodiment 2
[0042] Example 2: Synthesis of 5-(1-(2'-amino-5'-sodium formate phenyl)diazenyl)-1-naphthalenesulfonic acid sodium azo compound
[0043] At room temperature, take sodium nitrite (0.6900g, 0.01mol) and dissolve it in 10ml of distilled water to prepare an aqueous solution of sodium nitrite for subsequent use;
[0044] Dissolve 5-amino-1-naphthalenesulfonic acid (2.2325g, 0.01mol) and anhydrous sodium carbonate (1.6958g, 0.016mol) in 100ml of distilled water, then add sodium nitrite solution, stir for ten minutes to make it evenly mixed to form Mixture A;
[0045] Dilute concentrated hydrochloric acid (1.75ml, 0.02mol) into 1mol / L dilute hydrochloric acid. Under the condition of ice-water bath, add the mixed solution A dropwise into dilute hydrochloric acid, and form solution B after 10 minutes of reaction;
[0046] Under ice-water bath conditions, p-aminobenzoic acid (1.3714 g, 0.01 mol) and sodium hydroxide (0.8 g, 0.02 mol) were dissolved in 50 ml of distilled water to form ...
Embodiment 3
[0049] Example 3: Synthesis of 5-(4'-hydroxyphenyl)diazenyl)-1-naphthalenesulfonate sodium azo compound
[0050] At room temperature, take sodium nitrite (0.6900g, 0.01mol) and dissolve it in 10ml of distilled water to prepare an aqueous solution of sodium nitrite for subsequent use;
[0051] Dissolve 5-amino-1-naphthalenesulfonic acid (2.2325g, 0.01mol) and anhydrous sodium carbonate (1.0600g, 0.01mol) in 80ml of distilled water, then add sodium nitrite solution, stir for 10 minutes, and mix well Form a mixture A;
[0052] Dilute concentrated hydrochloric acid (1.75ml, 0.02mol) into 1mol / L dilute hydrochloric acid. Under the condition of ice-water bath, add the mixed solution A dropwise into dilute hydrochloric acid, and form solution B after 10 minutes of reaction;
[0053] Under ice-water bath conditions, phenol (0.9411 g, 0.01 mol), sodium hydroxide (0.8 g, 0.02 mol) and 20 ml of acetone were dissolved in 30 ml of distilled water to form solution C. At this temperature,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com