Application of trilobatin to cerebral ischemia-reperfusion injury preventing and treating drug
A technology of cerebral ischemia-reperfusion and trelobatin, which is applied in the field of pharmacy, can solve the problem of no anti-cerebral ischemia-reperfusion injury, achieve the effect of preventing and treating cerebral ischemia-reperfusion injury, good therapeutic effect, and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]A kind of application of trilobatin in preventing and treating cerebral ischemia-reperfusion injury medicine, take trilobatin 5g, medicinal starch 75g, microcrystalline cellulose 20g, medicinal starch is first dried, pass through 120 mesh sieves, and then Mix with trilobatin and microcrystalline cellulose, pass through a 120-mesh sieve twice, fill in hard capsules, and make 1000 capsules of the present invention; each hard capsule contains 0.5 mg of the main ingredient.
Embodiment 2
[0032] A kind of application of trilobatin in preventing and treating cerebral ischemia-reperfusion injury medicine, take trilobatin 5g, hypromellose 6g, carboxymethyl starch sodium 10g, microcrystalline cellulose 8g, lactose 115g, starch 50g, hard Magnesium stearate 1g; fully mix trelobatin with hypromellose, sodium starch glycolate, microcrystalline cellulose, lactose, starch, magnesium stearate and put it into a high-speed blender, spray with appropriate amount of water, Control it at 3-4%, then compress into tablets, make 1000 tablets, and coat with film.
Embodiment 3
[0034] A kind of application of trilobatin in the prevention and treatment of cerebral ischemia-reperfusion injury medicine, take trilobatin 5g, add 400 ml polyethylene glycol 200 to dissolve, then add appropriate amount of distilled water to dilute, then add appropriate amount of sucrose and adjust the volume to 1000 ml, stir well, filter, fill into 10 ml or 20 ml each, sterilized package.
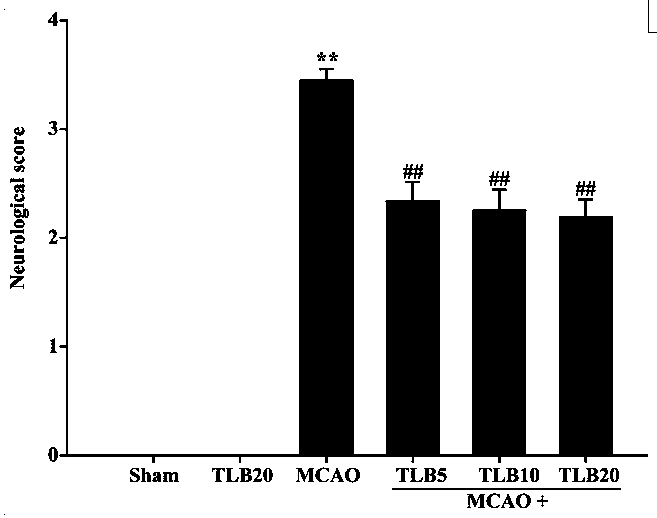
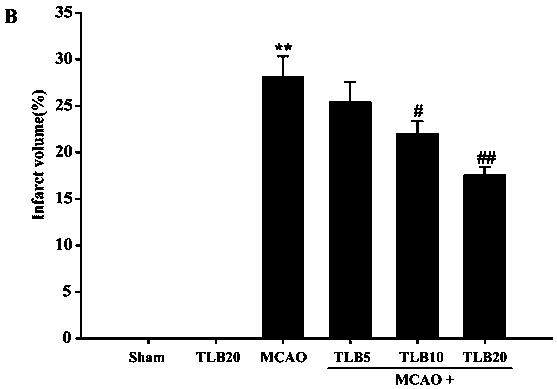
[0035] The invention adopts a rat model of focal cerebral ischemia-reperfusion injury to study the preventive effect of trilobatin on cerebral ischemia-reperfusion injury. Different concentrations of trilobatin (5, 10, 20 mg / kg) were given prophylactically for 7 days, and the MCAO model was prepared by suture method. After 2 hours of ischemia, reperfusion was performed for 24 hours. The results showed that trilobatin had a concentration-dependent preventive effect on cerebral ischemia-reperfusion injury, and significantly reduced the release of pro-inflammatory factors (TNF-α, IL-1β and I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com