Dapoxetine hydrochloride monohydrate and its preparation method and use
A technology for dapoxetine hydrochloride and monohydrate, which is applied in the field of dapoxetine hydrochloride monohydrate and its preparation, and can solve the problems of increasing preparation difficulty and production cost, unfavorable storage of raw materials and preparations, and affecting stability, etc. , to achieve the effect of solving the problem of metabolic rate, ensuring quality and efficacy, and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
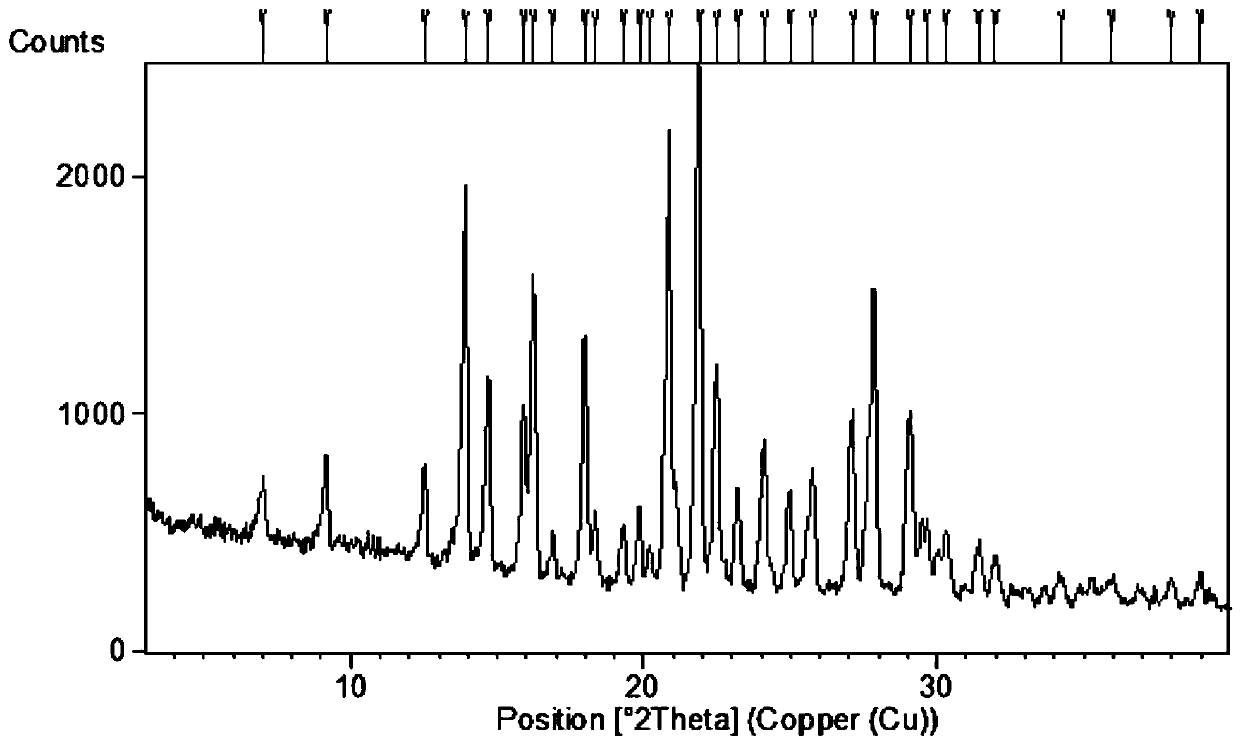
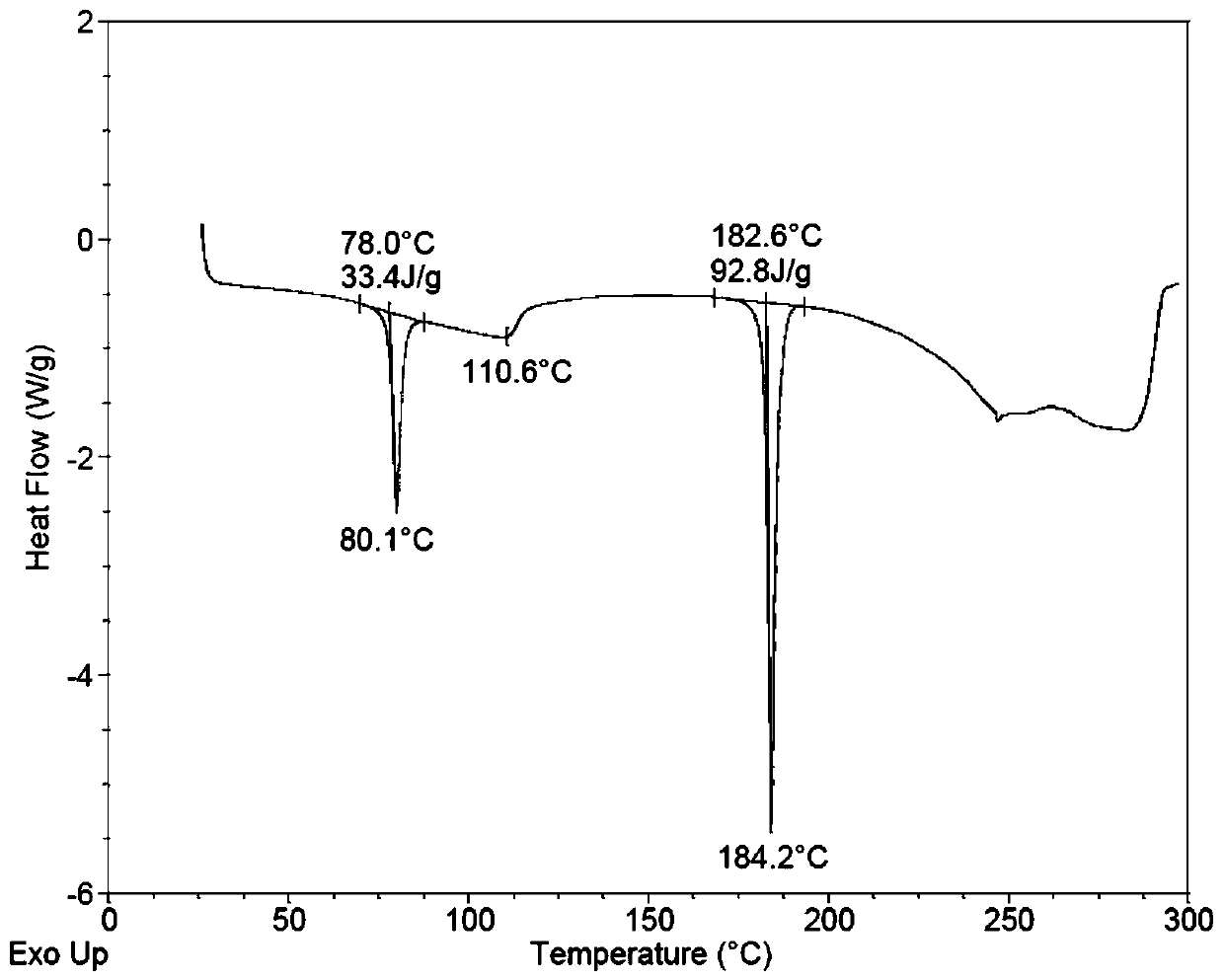
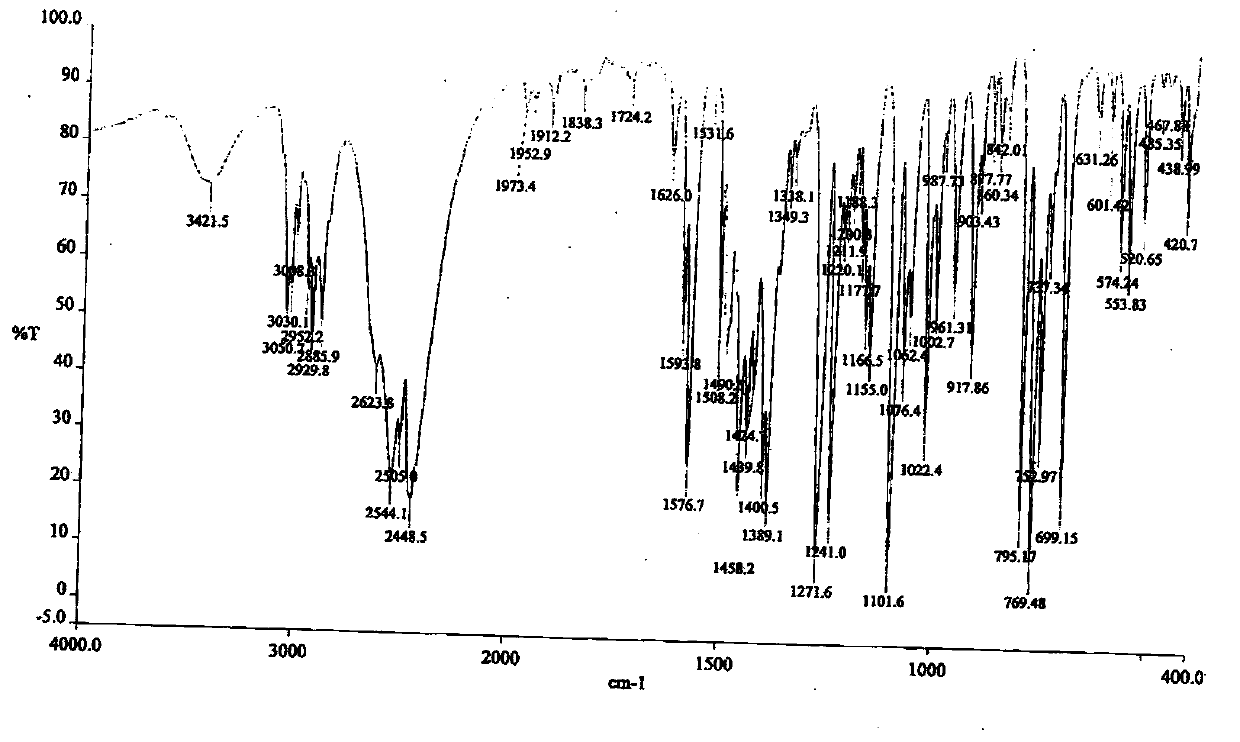
[0062] Spread 30.0 g of pulverized dapoxetine hydrochloride in a petri dish with a thickness of no more than 5mm; put the petri dish into a desiccator filled with saturated potassium nitrate aqueous solution (corresponding to 92.5% relative air humidity), Hydrate for seven days after sealing to obtain the crystal form of dapoxetine hydrochloride monohydrate. Sampling was carried out to determine moisture by Karl Fischer method, and the moisture content was 5.12%.
[0063] The results of elemental analysis show that the difference between the measured values of carbon, hydrogen and nitrogen in the product and the theoretical value is less than 0.3%, indicating that the molecular formula of the sample is the same as the molecular formula C of dapoxetine hydrochloride monohydrate. 21 h 26 ClNO 2 Contains the same amount of carbon, hydrogen, nitrogen elements.
[0064]
[0065] Note: "%" indicates the percentage of elemental molecular weight to the molecular weight of dapo...
Embodiment 2
[0073] Spread 5.0 g of pulverized dapoxetine hydrochloride in a petri dish with a thickness of no more than 1mm; put the petri dish into a desiccator filled with saturated potassium nitrate aqueous solution (corresponding to 92.5% relative air humidity), Hydrate for one day after sealing to obtain the monohydrate crystal form of dapoxetine hydrochloride. Sampling was carried out to determine moisture by Karl Fischer method, and the moisture content was 5.27%.
[0074] The detection spectrum and detection value of the obtained crystal characterization are basically the same as those in Example 1, indicating that the monohydrate crystal form of dapoxetine hydrochloride has been obtained.
Embodiment 3
[0076]Spread 5.0 g of pulverized dapoxetine hydrochloride in a petri dish with a thickness of no more than 1 mm; put the petri dish into a desiccator filled with saturated potassium iodide aqueous solution (equivalent to 67.8% relative air humidity), and seal After three days of hydration, the monohydrate crystal form of dapoxetine hydrochloride was obtained. Sampling was carried out by Karl Fischer method to determine the moisture, and the moisture content was 5.03%.
[0077] The detection spectrum and detection value of the obtained crystal characterization are basically the same as those in Example 1, indicating that the monohydrate crystal form of dapoxetine hydrochloride has been obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com