Preparation method for quick-release tablet cores of bupropion hydrochloride enteric-coated and sustained-release tablets
A technology of bupropion hydrochloride and sustained-release tablets, which is applied to medical preparations containing active ingredients, pharmaceutical formulas, coatings, etc., to achieve the effects of high application value, reduced material loss, and reduced time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
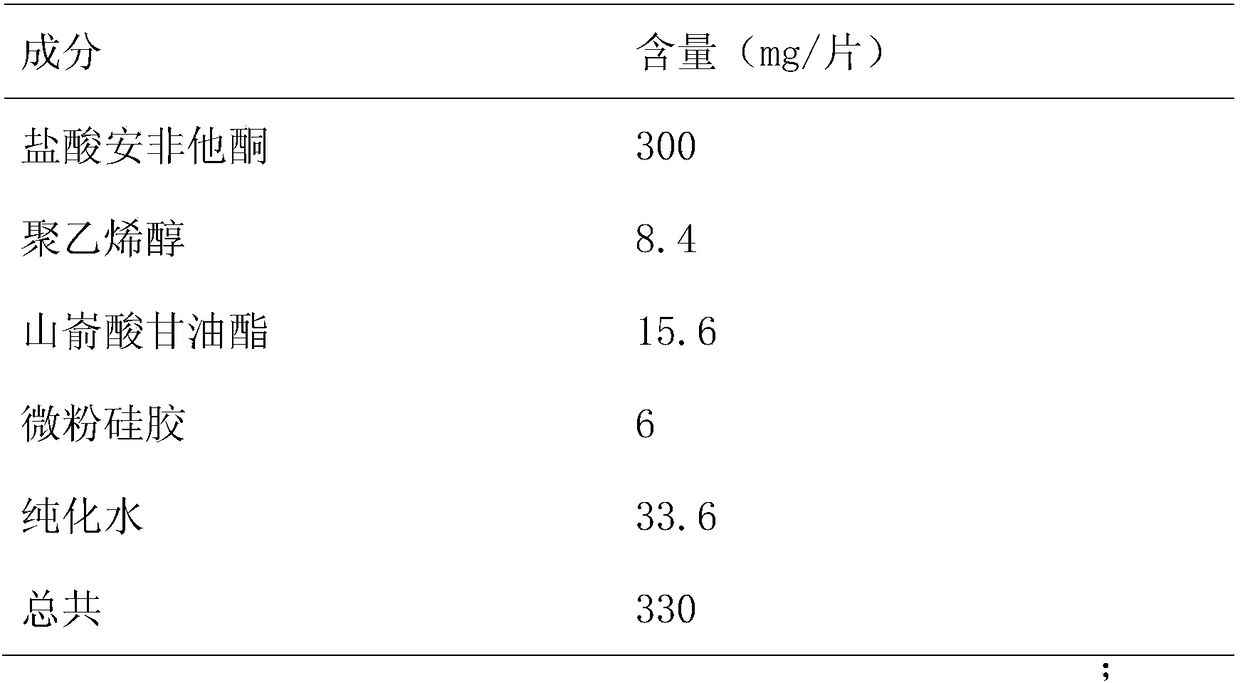
[0022] Formulation 1000 tablets in Table 1
[0023]
[0024] *Remove after drying
[0025] Dissolve polyvinyl alcohol in water to form a 20% concentration binder, place bupropion hydrochloride in a high-shear wet granulator (Mini-CG-1 / 10), add 20% binder Polyvinyl alcohol, stirred and cut evenly to make granules, sieved wet granules (1mm sieve), dried (dried in oven at 60°C for 1 hour), granulated, added micro-powdered silica gel and mixed for 20 minutes, then added glyceryl behenate, mixed evenly, and pressed into tablets 600 immediate-release cores of bupropion hydrochloride sustained-release tablets were prepared, with a tablet weight of 320mg±3%
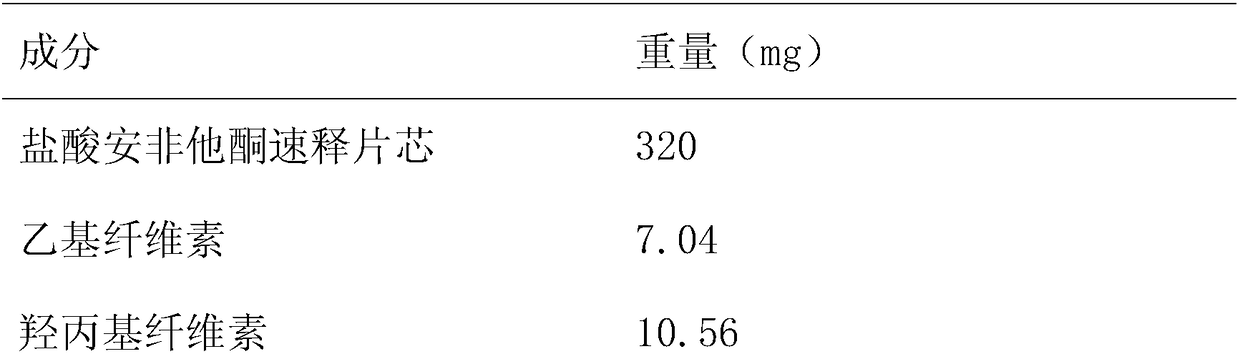
[0026] Formulation 500 tablets in Table 2
[0027]
[0028]
[0029] Get each raw and auxiliary material according to Table 2, add ethyl cellulose and hydroxypropyl cellulose in ethanol under stirring state, be made into coating solution, then make the immediate release of bupropion hydrochloride sustained-release tabl...
Embodiment 2
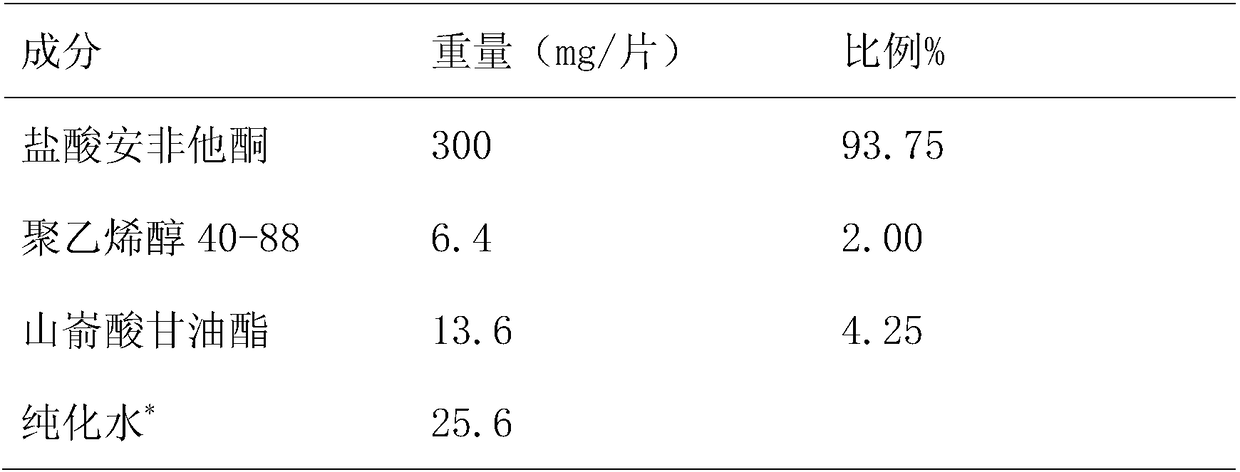
[0033] Embodiment 2: prepare 1000 tablets
[0034] Table 4
[0035]
[0036] *Remove after drying
[0037] Take the raw and auxiliary materials according to Table 4, place bupropion hydrochloride in a high-shear wet granulator, add 20% binder polyvinyl alcohol, stir to make granules, and sieve the wet granules (1mm sieve) After drying (drying in an oven at 60° C. for 1 hour), glyceryl behenate was added and mixed evenly, and tableted to obtain 600 tablet cores with a tablet weight of 320 mg±3%. The core of the tablet is coated with a slow-release layer, and the weight gain of the coating is 4%-7%, and the sustained-release tablet is further coated with an enteric layer to obtain a bupropion hydrochloride sustained-release tablet, and the weight gain of the coating is 1.5%-3.5%.
Embodiment 3
[0039] table 5
[0040]
[0041] *Remove after drying
[0042] Weigh raw and auxiliary materials according to Table 5, place bupropion hydrochloride in a high-shear wet granulator, add 20% binder polyvinyl alcohol, stir to make granules, and sieve the wet granules (1mm sieve) After drying (drying in an oven at 60° C. for 1 hour), glyceryl behenate was added and mixed evenly, and tableted to obtain 600 tablet cores with a tablet weight of 320 mg±3%. The core of the tablet is coated with a slow-release layer, and the weight gain of the coating is 4%-7%, and the sustained-release tablet is further coated with an enteric layer to obtain a bupropion hydrochloride sustained-release tablet, and the weight gain of the coating is 1.5%-3.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com