Florfenicol and thiamphenicol antigens and antibodies as well as enzyme-linked immunosorbent assay method for detecting florfenicol and thiamphenicol simultaneously
A technology of florfenicol and thiamphenicol, which is applied in the field of antibody and its simultaneous detection of enzyme-linked immunoassay, florfenicol and thiamphenicol antigen, and can solve the problems of expensive equipment and low sample throughput.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The preparation method of embodiment 1 hapten
[0083] 1. Preparation of Hapten FFD
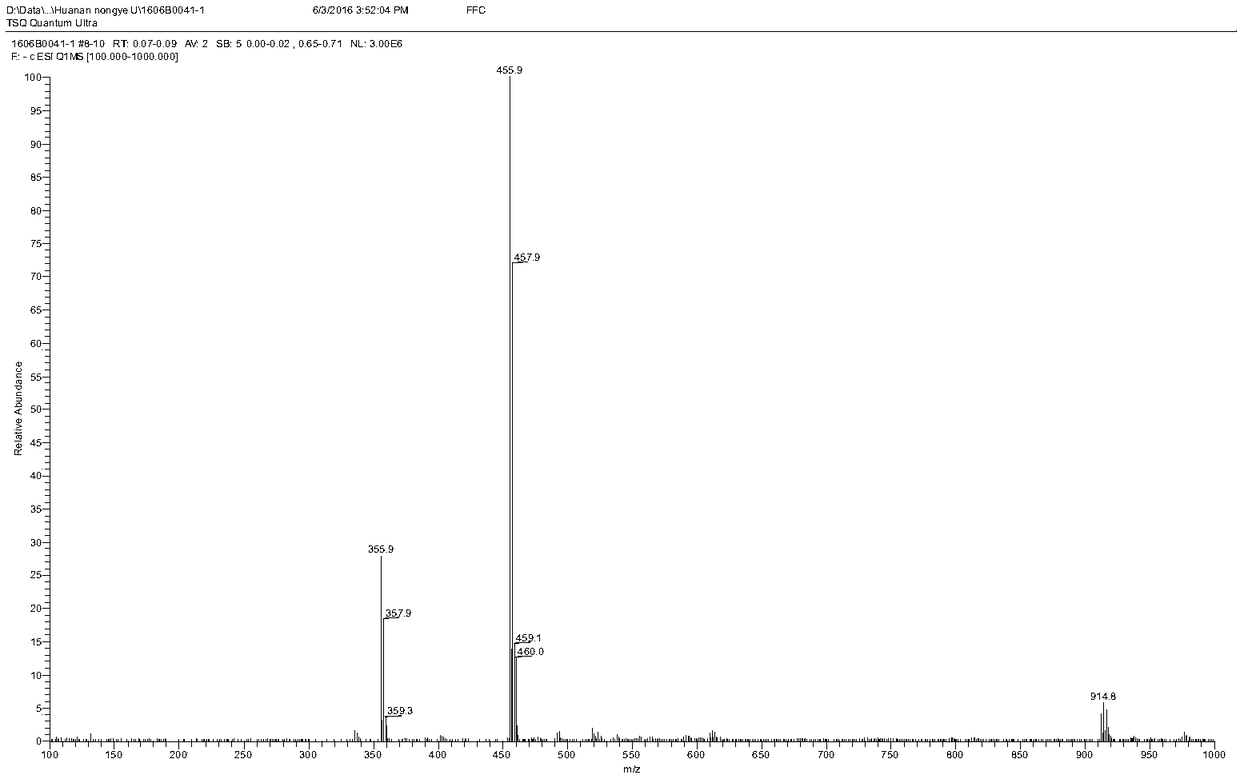
[0084] Add 580 mg of florfenicol and 240 mg of succinic anhydride into 5 ml of dichloromethane, then add 680 μL of triethylamine dropwise to the above mixture, and heat the reaction in a water bath at 50°C for 3 hours. After the reaction, the product mixture is washed with ethyl acetate The ester was extracted 3 times, and the volume ratio of each extraction mixture to ethyl acetate was 1:2; the extract was spin-dried and purified through a silica gel column, and after evaporation to dryness, a light yellow powder was obtained, which was the hapten FFD. figure 1 It is the identification map of FFD mass spectrum. 2. Preparation of Hapten FFM
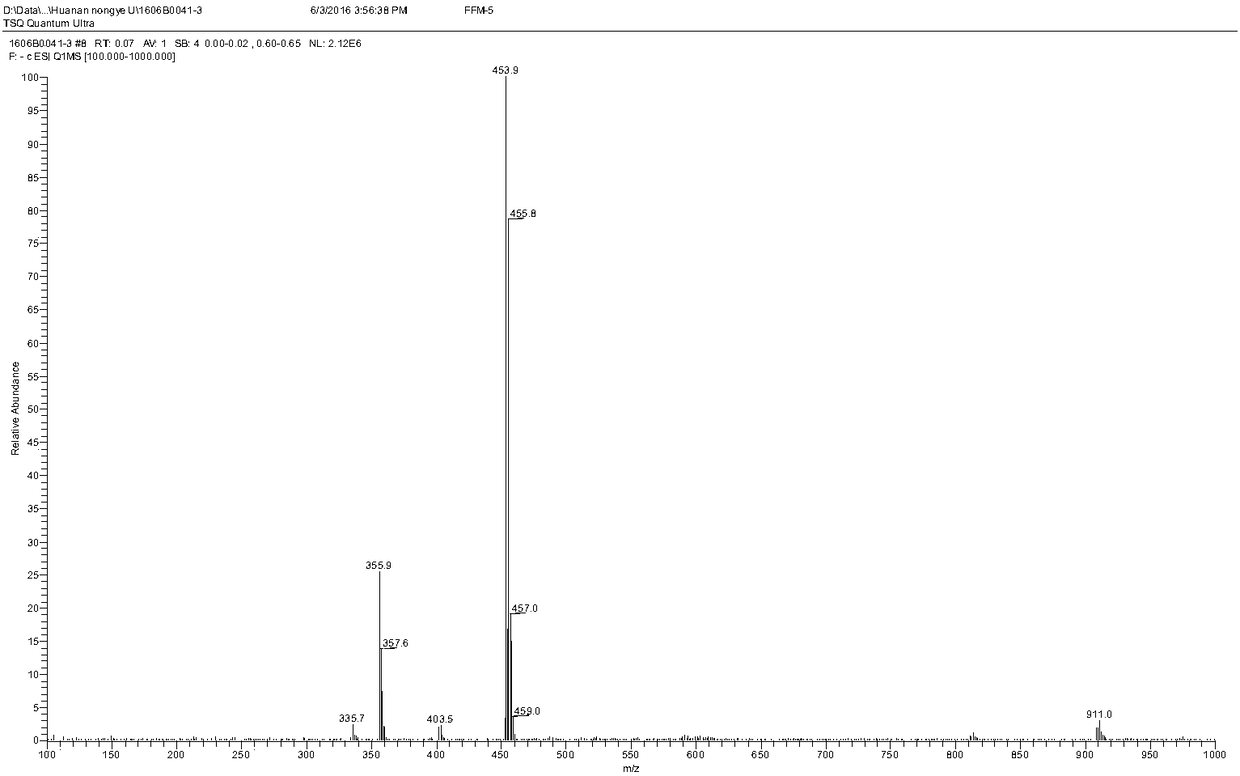
[0085] Add 580 mg of florfenicol and 240 mg of maleic anhydride to 5 ml of dichloromethane, then add 680 μL of triethylamine dropwise to the above mixture, and heat the reaction in a water bath at 50°C for 3 hours. After the reaction, the produc...
Embodiment 2
[0086] The preparation of embodiment 2 immunogen and coating former
[0087] 1. Preparation of Immunogen FFD-KLH
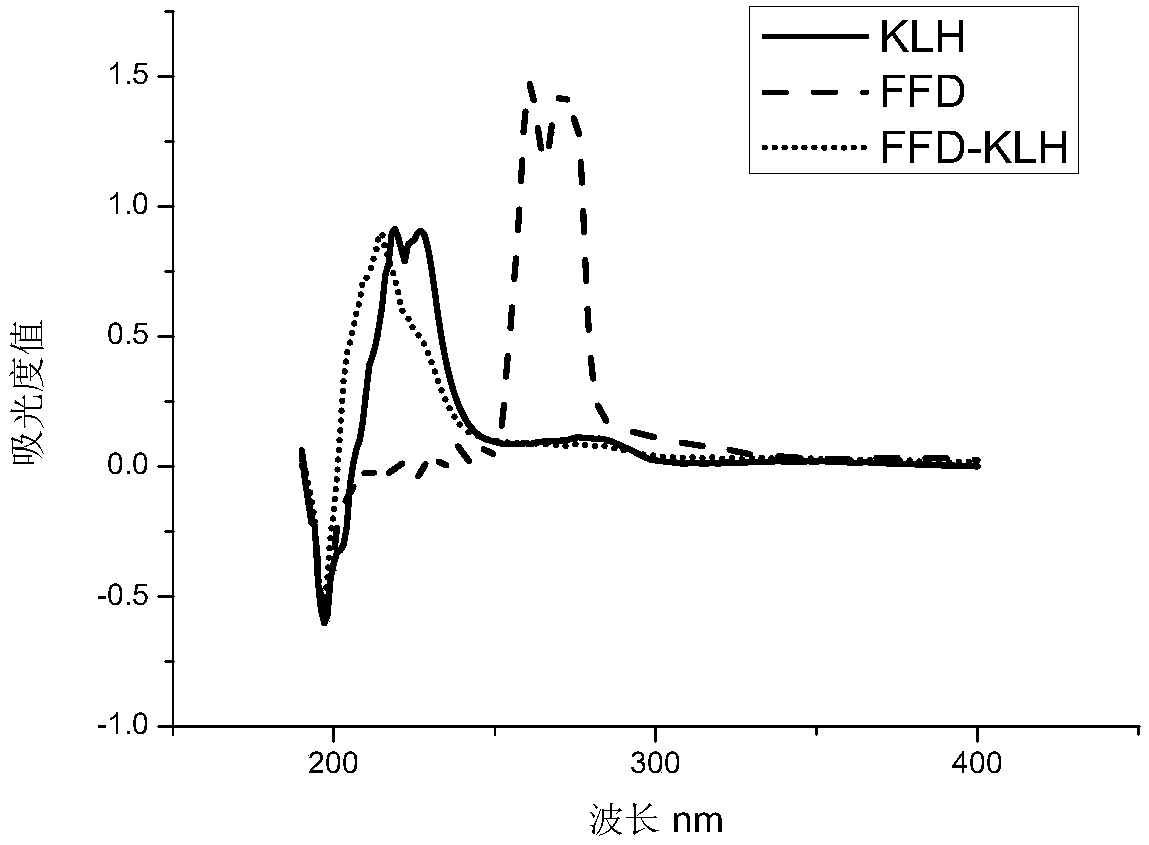
[0088] Active lipid method: Dissolve 5mg FFD hapten in 0.2mL N,N-dimethylformamide, then add 4.78mg N,N-dicyclohexylcarbodiimide and 2.65mg N-hydroxysuccinimide , stirred and reacted at 4°C overnight, and the supernatant was taken after centrifugation and recorded as solution A. Dissolve the carrier protein (14.6mg KLH) in 4mL 0.01mol / L PBS, record it as solution B, drop solution A into solution B under stirring, react at 4°C for 12h, centrifuge after the reaction, take the supernatant, put in PBS at 4°C The solution was dialyzed for 3 days to obtain the florfenicol artificial immunogen, which was dispensed into 1 mL centrifuge tubes at a concentration of 1 mg / mL, and frozen in a -20°C refrigerator for later use.
[0089] 2. Preparation of coated raw FFM-OVA
[0090] Active lipid method: Dissolve 10mg FFM hapten in 0.2mL N,N-dimethylformamide; add 9.56mg N,N-di...
Embodiment 3
[0094] Example 3 Antibody Preparation and Identification
[0095] When 2.5kg female New Zealand white rabbits were immunized for the first time, the amount of immunogen injected was 0.5mL / rat. Take 0.5mL 1mg / mL immunogen plus an equal volume of Freund's complete adjuvant. Spot injection, about 200 μl per spot; the second immunization was carried out four weeks later, the amount of injected immunogen was 0.5mL / monkey, emulsified with an equal volume of Freund's incomplete adjuvant; the third immunization was carried out three weeks later, the fourth There was also an interval of 3 weeks between immunization and the third time, and a total of 4 times of immunization. 4 One week after immunization, blood was collected from the heart to collect antibody serum. The antiserum was purified by caprylic acid-ammonium sulfate precipitation method to obtain polyclonal antibody, and stored at -20°C for future use.
[0096] On the seventh day after the third booster immunization, 200 μL o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com