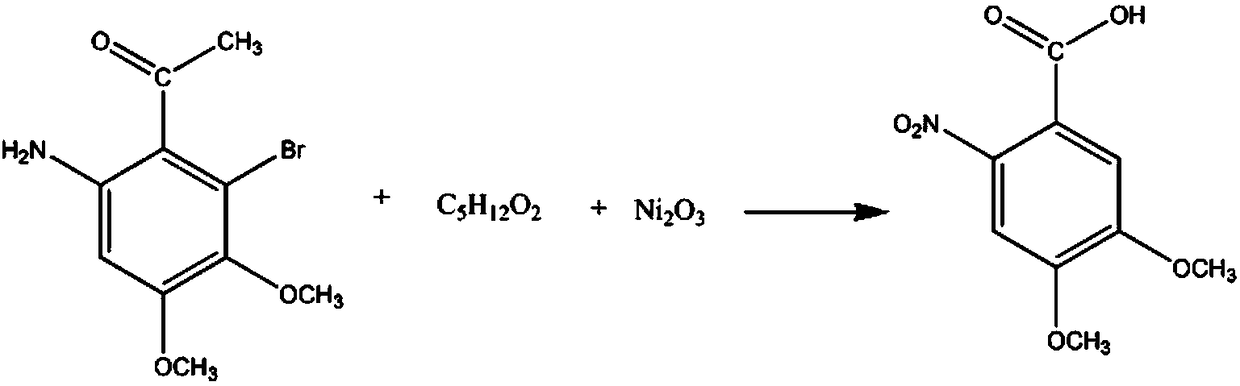Pharmaceutical intermediate 3,4-dimethoxy-6-nitrobenzoic acid synthesis method
A technology of nitrobenzoic acid and dimethoxy, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve problems such as complex process and low final yield, and reduce intermediate links , shorten the reaction time, improve the effect of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0010] Add 3mol of 2-bromo-3,4-dimethoxy-6-aminoacetophenone and 6mol of 1,5-pentanediol solution into the reaction vessel, control the stirring speed at 210rpm, keep it for 30min, add 600ml mass fraction 10% potassium carbonate solution, adjust the pH to 8, increase the solution temperature to 60°C, add 3mol of nickel oxide, continue the reaction for 30min, add a 20% potassium hydrogensulfate solution by mass fraction, adjust the pH to 2, Reduce the solution temperature to 10°C, wash with 30% sodium sulfate solution in mass fraction, wash with 75% methylcyclohexanone solution, wash with 80% 1-methylnaphthalene solution with 90% mass fraction % of 2-methyl-3-pentanol solution and dehydrated with anhydrous potassium carbonate dehydrating agent to obtain 606.09 g of finished product 3,4-dimethoxy-6-nitrobenzoic acid with a yield of 89%.
example 2
[0012] Add 3mol of 2-bromo-3,4-dimethoxy-6-aminoacetophenone and 7mol of 1,5-pentanediol solution into the reaction vessel, control the stirring speed at 230rpm, keep it for 35min, add 600ml mass fraction For 13% potassium carbonate solution, adjust the pH to 8.5, increase the temperature of the solution to 65°C, add 4mol of nickel oxide, continue the reaction for 35min, add a 23% potassium hydrogensulfate solution by mass fraction, adjust the pH to 2.5, Lower the solution temperature to 12°C, wash with 32% sodium sulfate solution in mass fraction, wash with methylcyclohexanone solution with 79% mass fraction, wash with 1-methylnaphthalene solution with 83% mass fraction, and wash with 93% mass fraction % of 2-methyl-3-pentanol solution and dehydrated with anhydrous magnesium sulfate dehydrating agent to obtain 626.52 g of finished product 3,4-dimethoxy-6-nitrobenzoic acid with a yield of 92%.
example 3
[0014] Add 3mol of 2-bromo-3,4-dimethoxy-6-aminoacetophenone and 8mol of 1,5-pentanediol solution into the reaction vessel, control the stirring speed at 250rpm, keep it for 40min, add 600ml mass fraction For a 17% potassium carbonate solution, adjust the pH to 9, increase the temperature of the solution to 70°C, add 5mol of nickel oxide, continue the reaction for 40min, add a 26% potassium bisulfate solution by mass fraction, adjust the pH to 3, Lower the solution temperature to 15°C, wash with 35% sodium sulfate solution in mass fraction, wash with 82% methylcyclohexanone solution, wash with 86% 1-methylnaphthalene solution, and wash with 97% mass fraction % of 2-methyl-3-pentanol solution and dehydrated with anhydrous calcium sulfate dehydrating agent to obtain 653.76 g of finished product 3,4-dimethoxy-6-nitrobenzoic acid with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com