Antigenic peptide chain group for treating tumor and application thereof to medicine
A technology of antigenic peptides and drugs, applied in the field of malignant tumor bioimmune drugs, can solve problems such as difficult side effects and non-specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Patient A, male, 63 years old. Lung adenocarcinoma, clinical stage IV, progressed after 5 cycles of vinorelbine combined with cisplatin chemotherapy, with multiple lung metastases. A lung lesion biopsy was taken, and 488 tumor occurrence and development-related gene panels and HLA typing chips were tested. The patient carried the EGFR C797S mutation, and the HLA typing was HLA-A: A*1002A*3102; HLA-B: B* 3101B*4013; HLA-C: C*0303C*0801; HLA-DQB1: DQB1*0402DQB1*0101; HLA-DRB1: DRB1*0405DRB1*1301.
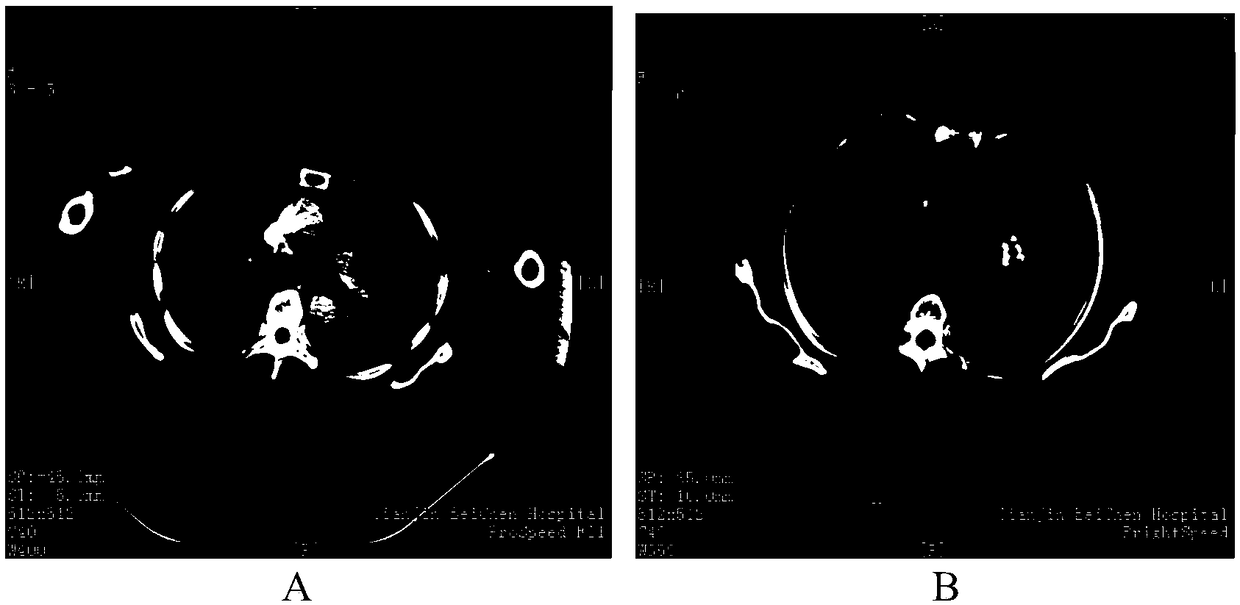
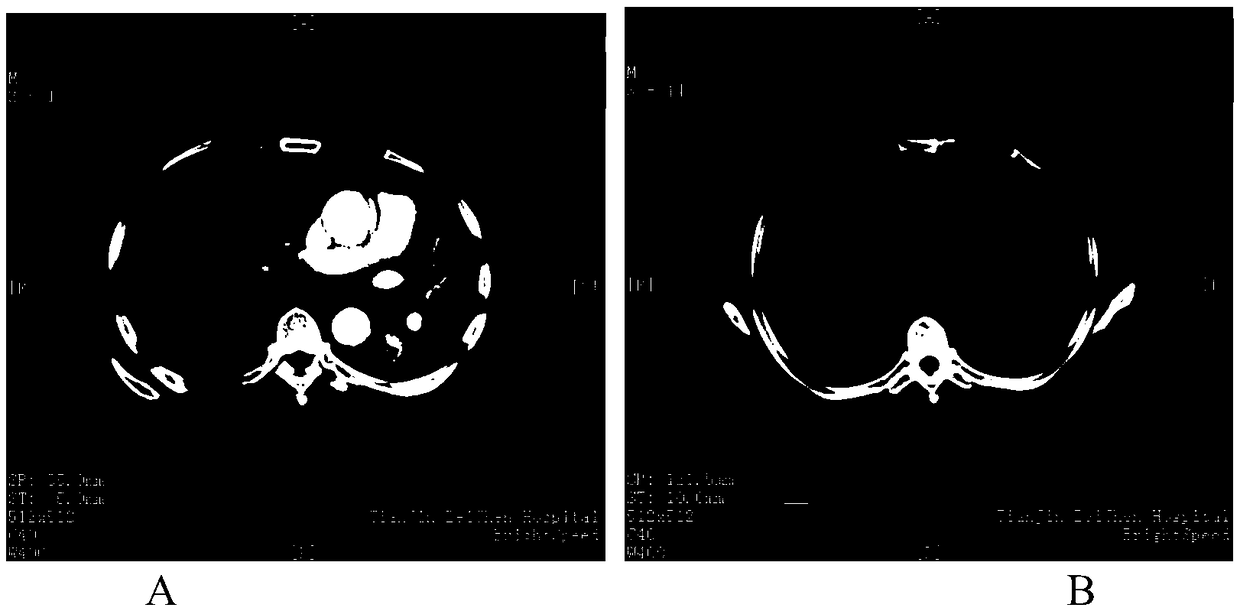
[0065] Combination therapy (hereinafter referred to as "administration") was performed using the antigen peptide chain group for treating tumors of the present invention, once a week for a total of 10 weeks. Flow detected the specific IFN-γ secretion before, after 3 weeks, and after 10 weeks of medication, and observed the changes in tumor size with imaging at the same time points (see Figure 1 and Figure II ). The specific selected peptide chains are as follows:
[0066...
Embodiment 2
[0070] Patient B, female, 51 years old, with adenocarcinoma of the left lung, progressed after 5 cycles of chemotherapy and oral molecular targeted drug gefitinib. A lung tumor biopsy was performed, and 488 tumor occurrence and development-related gene panels and HLA typing chip tests were performed. The results showed that the patient carried the EGFR C797S mutation. HLA typing is HLA-A: A*0302A*3305; HLA-B: B*1801B*4002; HLA-C: C*0301C*0401:; HLA-DQB1: DQB1*0102DQB1*0203; HLA-DRB1: DRB1 *0301DRB1*1004.
[0071] Combination therapy was performed with the antigen peptide chain group for treating tumors of the present invention once a week for a total of 12 weeks. The specific selected peptide chains are as follows:
[0072] a) PFGSLLD, b) LMPFGSLL, c) GSLLDYVR, d) QLMPFGSLL, e) GSLLDYVRE, f) TVQLMPFGSL, g) FGSLLDYVRE, h) VQLMPFGSLLD, i) LTSTVQLMPFGS, j) GSLLDYVREHKD.
[0073] The changes in the size of lung tumors before and after treatment are as follows: image 3 shown b...
Embodiment 3-60
[0075] Example 3-60 Cancer patients of different types and degrees were taken, and 488 tumor occurrence and development-related gene panels and HLA typing chip tests were performed. All patients carried the EGFR p.C797S mutation, and different antigen peptide chain groups were used The combined form of treatment was given once a week for 12 weeks. The results of HLA*A3101-HVKITDFGR Tetramer staining and the change of lung tumor size before and after treatment in Example 3-60 are similar to those in Example 1-2, and will not be repeated here due to space limitations. The above results can all show that the antigen peptide chain group of the present invention can induce dendritic cells that produce tumors, and dendritic cells as antigen-presenting cells can present antigen information to T cells, interact with T cells, and cause T cells to produce tumors. It specifically kills tumor cells, plays a role in killing tumor cells, and the effect is obvious.
[0076] During the treat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com