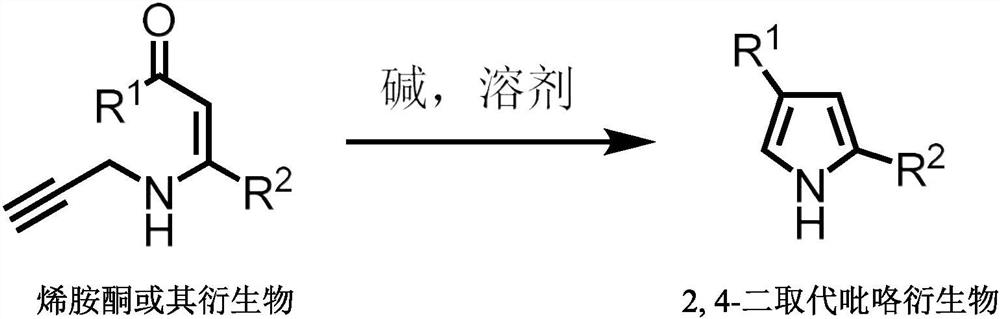
A kind of preparation method of 2,4-disubstituted pyrrole derivatives
A technology for disubstituted pyrroles and derivatives, applied in 2 fields, can solve the problems of low universality, numerous and complicated operation steps, etc., and achieve the effects of strong reaction specificity, wide substrate range and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of 2,4-diphenylpyrrole
[0022]
[0023] Add (Z)-1,3-diphenyl-3-(N-propargylamino)prop-2-en-1-one 0.5mmol, potassium carbonate 0.6mmol, N,N-dimethylacetamide 5mL In a 10mL reaction tube, place in an oil bath at 140°C and react for 24h. The reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate, and purify by column chromatography to obtain 99.1 mg of the target product with a yield of 90%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, DMSO) δ11.44(s, 1H), 7.69(d, J=7.8Hz, 2H), 7.62(d, J=7.7Hz, 2H), 7.35(m, 5H), 7.15(m , 2H), 6.96(s, 1H); 13 C NMR (100 MHz, DMSO) δ 136.2, 133.1, 132.7, 129.1, 129.0, 126.1, 125.5, 125.2, 124.9, 123.9, 117.0, 103.6.
Embodiment 2
[0025] Preparation of 2-phenyl-4-(p-tolyl)pyrrole
[0026]
[0027] (Z)-1-(p-tolyl)-3-phenyl-3-(N-propargylamino)prop-2-en-1-one 0.5mmol, potassium carbonate 0.6mmol, N,N-dimethyl Add 5mL of acetamide into a 10mL reaction tube, place in an oil bath at 140°C, and react for 24h. The reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate, and purify by column chromatography to obtain 108.3 mg of the target product with a yield of 93%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )68.40(s, 1H), 7.48(dd, J=17.2, 7.9Hz, 4H), 7.38(t, J=7.6Hz, 2H), 7.22(d, J=7.4Hz, 1H), 7.17(d, J=7.8Hz, 2H), 7.10(s, 1H), 6.80(s, 1H), 2.36(s, 3H); 13 C NMR (100MHz, CDCl 3 ) δ 135.2, 132.9, 132.5, 129.3, 128.9, 126.6, 126.4, 125.1, 123.8, 115.2, 103.9, 21.0.
Embodiment 3~13
[0029] The operation steps are the same as in Example 2, the difference is: according to the following reaction formula, the substituent R of enaminone is changed 1 and R 2 , to obtain different 2,4-disubstituted pyrrole derivatives.
[0030]
[0031] The reaction temperature in this example is 140°C.
[0032] The structural characterization data of some products are as follows:
[0033] Characterization data for product 2a: 1 H NMR (400MHz, CDCl 3 )δ8.44(s, 1H), 7.50(d, J=7.8Hz, 2H), 7.38(t, J=7.6Hz, 2H), 7.30-7.20(m, 2H), 7.17(d, J=7.6 Hz, 1H), 7.11(s, 2H), 6.84-6.72(m, 2H), 3.85(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ159.9, 136.9, 133.0, 132.4, 129.6, 128.9, 126.5, 123.8, 117.8, 115.7, 111.1, 110.9, 104.0, 55.2; HRMS (ESI) calcd for C 17 h 16 NO[M+H]+250.1226, found: 250.1232.
[0034] Characterization data for product 2b: 1 H NMR (400MHz, DMSO) δ11.44(s, 1H), 7.69(d, J=7.9Hz, 2H), 7.64(dd, J=7.5, 5.7Hz, 2H), 7.38(t, J=7.4Hz , 2H), 7.32(s, 1H), 7.22-7.12(m, 3H), 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com