Luminescent material with thermotropic delayed fluorescence, application of luminescent material and electroluminescent device
A technology of thermally induced delayed fluorescence and luminescent materials, which is applied in the field of electroluminescent devices, can solve the problems of few electron-donating groups and electron-withdrawing groups, difficulty in molecular design of thermally induced delayed fluorescent materials, etc., and achieve long service life, Realize the effect of deep blue light emission and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The spirobifluorene indole derivative 13 (5-(4,6-diphenyl-1,3,5-triazine)-5H spirofluorene-9,8-indenecarbazole) of the present invention can be Synthesized by the following method.
[0069]
[0070] (1) In a dry 500mL two-necked flask, 3-carbazoleboronic acid pinacol ester (15g, 51mmol), o-dibromobenzene (14.5g, 61.2mmol), toluene (120mL), ethanol (60mL) and 2mol / L potassium carbonate solution (60mL) were added, ultrasonication was performed first, and then nitrogen gas was rapidly stirred, and the catalyst tetrakis(triphenylphosphine)palladium (1.8g, 1.53mmol) was added rapidly, and a large amount of nitrogen gas was passed. Heat to 90°C and stir overnight. During treatment, extract first, spin dry, and use petroleum ether and dichloromethane column chromatography to obtain the white solid product 3-(2-bromophenyl)-9H-carbazole with a yield of 93%. C 18 h 12 BrN, mass spectrometry theoretical value 321.0153, measured by APCI (atmospheric pressure chemical ioniza...
Embodiment 2
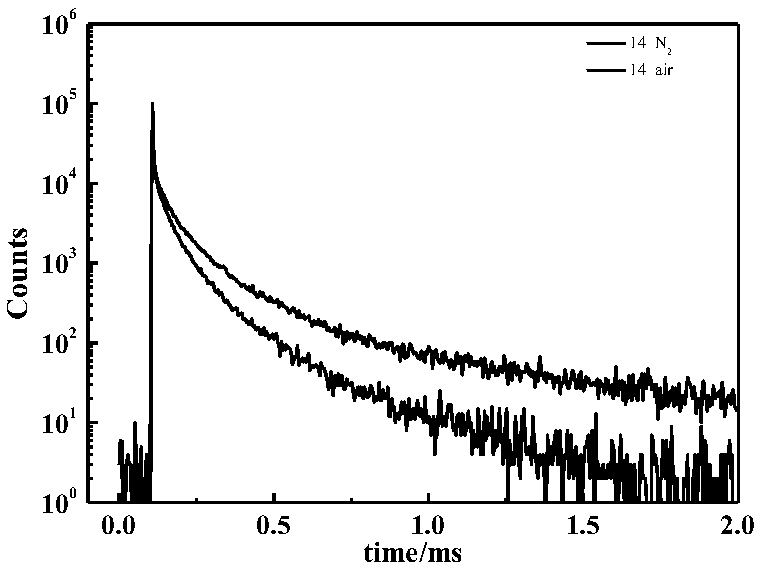
[0079] The carbazole derivative prepared in Example 1 has the formula 13,5-(2-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-12,12- Diphenyl-5,12-dihydroindeno[1,2-c]carbazole was used as light-emitting guest to prepare devices.
[0080]This example demonstrates the performance verification of an electroluminescent device prepared with 13 as a guest luminescent material. The ITO (Indium Tin Oxide) glass was ultrasonically cleaned in detergent and deionized water for 30 minutes sequentially. Then vacuum dry for 2 hours (105°C), then put the ITO glass into the plasma reactor for 5 minutes of oxygen plasma treatment, transfer it to the vacuum chamber to prepare organic film and metal electrode, and then prepare a layer of 10nm by vacuum evaporation. The hole injection material molybdenum trioxide, followed by evaporation of 70nm thick hole transport material: N4, N4'-two (naphthalene-1-yl) -N4, N4'-diphenyl-[1,1'-bi Benzene]-4,4'-diamine (NPB), then vapor-deposit 1,3-bis(9-carbazolyl...
Embodiment 3
[0083] 14(5-(3-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-12,12-diphenyl-5,12- Dihydroindeno[1,2-c]carbazole) can be synthesized by the following method.
[0084]
[0085] 14(5-(3-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-12,12-diphenyl-5,12-dihydroindeno[ 1,2-c]carbazole) synthesis. First synthesize the obtained 12,12-diphenyl-5,12-dihydroindeno[1,2-b]carbazole (1g, 2.466mmol) according to the steps (1)~(4) of Example 1, 2, 4-diphenyl-6 (3-bromophenyl) triazine (1.15g, 2.95mmol), cuprous iodide (0.28g, 1.47mmol), K 2 CO 3 (2.04g, 14.7mmol) and 18-crown-6 (0.378g, 1.47mmol) were dissolved in 3 mL of DMPU solution. Under the protection of N2, the temperature was raised to 175° C., and the reaction was carried out for 36 hours. After the reaction was terminated, it was cooled to room temperature, extracted, spin-dried, and subjected to column chromatography. Column chromatography with petroleum ether and dichloromethane gave 1.2 g of a yellow solid product with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com