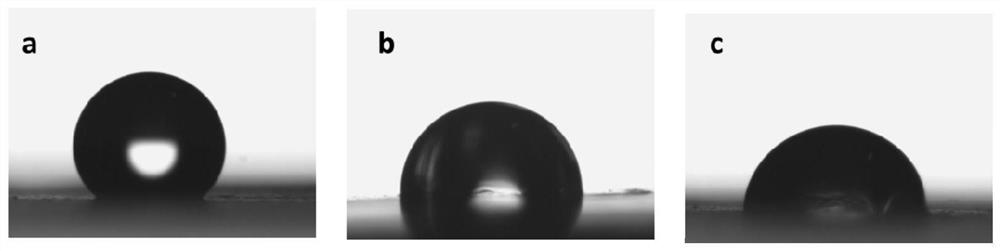
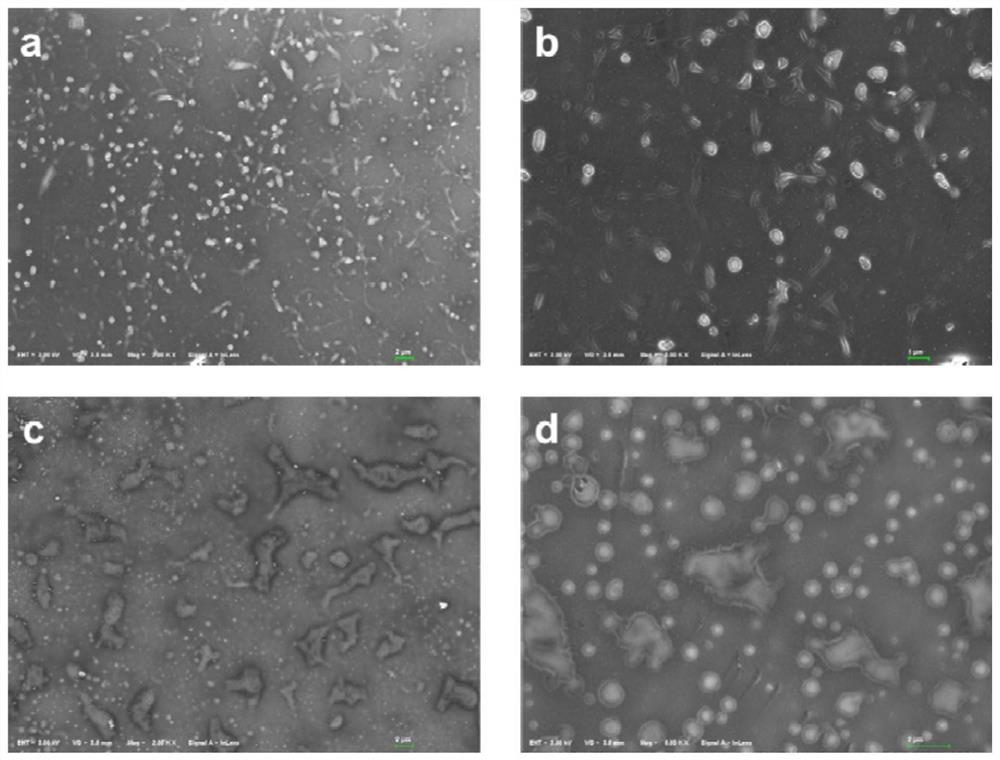
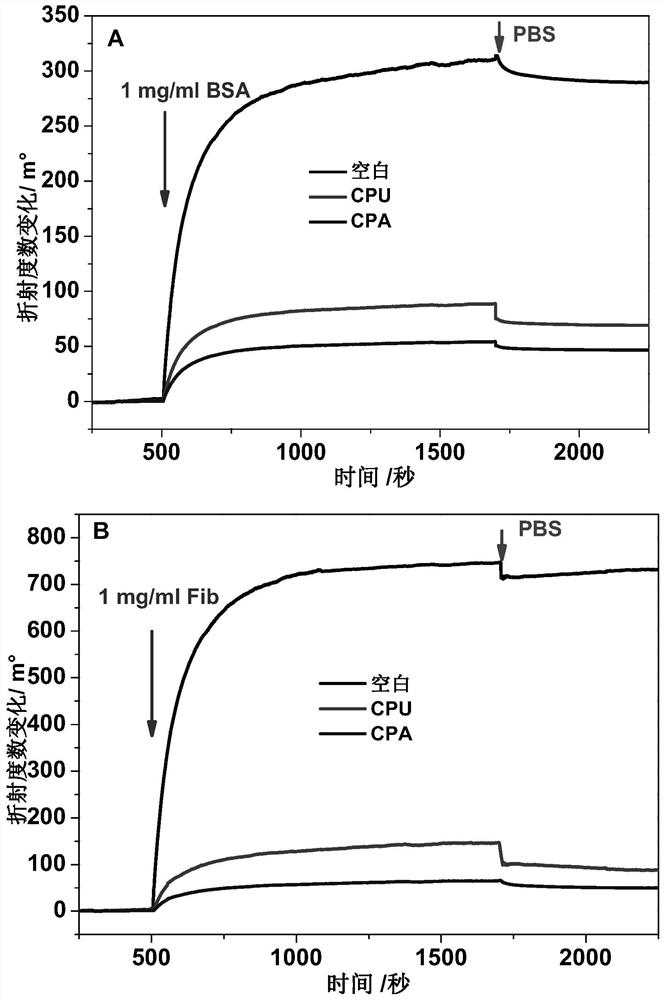
Synthesis and application of catechol derivatives and their biomimetic polymers
A technology of catechol and derivatives, which is applied in the synthesis field of catechol derivatives and biomimetic polymers, can solve the need for cumbersome protection and deprotection of phenolic hydroxyl groups, and catechol compounds cannot be synthesized with biomimetic polymers , long synthetic route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment 1 pyrocatechol derivatives
[0027] The general method for the synthesis of catechol derivatives: add secondary amine (0.03mol), formaldehyde solution (37% solution, 0.03mol), 50mL water in a 150mL flask, stir and react for 30min at 25°C, N 2 Under atmosphere, an aqueous solution (10 mL) of catechol (0.03 mol) was slowly added, and the reaction was continued for 3 h, and the end point of the reaction was monitored by TLC. Dilute hydrochloric acid (1 mol / L) was added to the reaction solution to adjust the pH value of the reaction solution to about 2, and the raw material of catechol was recovered by extraction with ether, and the water phase was retained. Add NaOH solution to adjust the pH value of the aqueous phase to about 8, extract 3 to 5 times with 100 mL ethyl acetate, and remove the organic phase with anhydrous Na 2 SO 4 Drying, precipitation, recrystallization from an appropriate amount of acetonitrile to obtain the 4-substituted cat...
Embodiment 2
[0039] The synthesis of embodiment 2 polyurethane biomimetic polymer (CPU)
[0040] In a 100mL three-necked flask, add polyethylene glycol (molecular weight 1000, 4.0g, 4mmol) and 20mL N,N-dimethylformamide (DMF, anhydrous) that were dewatered under vacuum at 100°C, and heat up to 70°C. N 2 With mechanical stirring, slowly add isophorone diisocyanate (1.78g, 8mmol) dropwise, react for 2h, control -NCO content at 5.8% of theoretical value (use di-n-butylamine method for titration, bromophenol blue as indicator) , cooled to room temperature, slowly added dropwise 5 mL of a DMF solution containing 4-(N,N-bis(2-hydroxyethyl)aminomethyl)catechol (II-c) (0.91g, 4mmol), 60°C Reaction 2h. Cool to room temperature, add anhydrous diethyl ether to precipitate, centrifuge to obtain a yellow thick or jelly, and vacuum dry at 40°C for 48 hours to obtain catechol-containing biomimetic polyurethane CPU with a yield of 85%.
[0041] 1 H NMR (600MHz, DMSO-d6) δ6.6-7.1 (broad peak, benzene r...
Embodiment 3
[0042] The synthesis of embodiment 3 polyacrylate biomimetic polymer (CPA)
[0043] First, the methacrylated intermediate II-d (catechol-containing acrylate) was synthesized. 4-(N-(2-hydroxyethyl)--N-methylaminomethyl)catechol (II-d, 1.97g, 10mmol) was dissolved in 50mL of anhydrous tetrahydrofuran, and triethylamine ( 2.02g, 10mmol), slowly dropwise added 10mL of anhydrous tetrahydrofuran solution containing 2-methacrylic anhydride (2.31g, 15mmol) under ice-cooling, and reacted at room temperature for 12h. After column chromatography (eluent: ethyl acetate / petroleum ether, volume ratio 1 / 2), 2-(N-(3,4-dihydroxybenzyl)-N-methylamino)ethylmethyl Acrylate (yellow oil, 1.91 g, yield 72%). 1 H NMR (600MHz, CDCl 3 )δ6.76(s,1H),6.71(d,J=7.9Hz,1H),6.63(d,J=7.5Hz,1H),6.18(s,2H),6.10(s,1H),5.56( s, 1H), 4.31 (t, J = 5.6Hz, 2H), 3.48 (s, 2H), 2.76 (t, J = 5.6Hz, 2H), 2.31 (s, 3H), 1.92 (s, 3H).
[0044] In a three-necked flask, add methacrylation intermediate II-d (1.33g, 5mmol), p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com