Method for synthesizing rivaroxaban process impurity
A technology of process impurities, rivaroxaban, applied in the direction of organic chemistry, etc., to achieve the effects of easy availability of raw materials, simple operation and enhanced control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
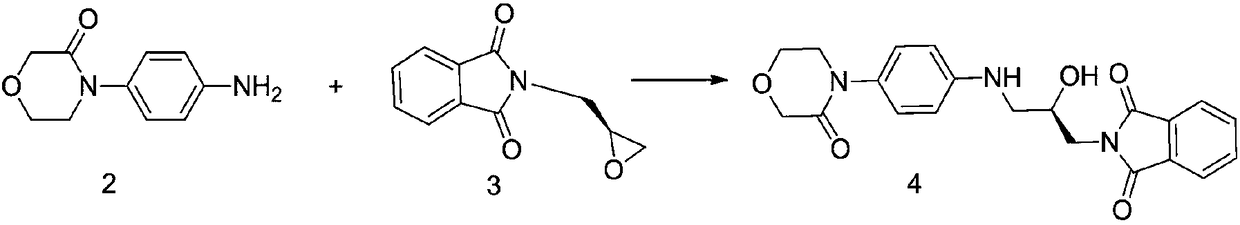
[0015] 2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-morpholine)phenyl]amino]propyl]-1H-isoindole-1,3(2H)- Preparation of diketone (4)
[0016]
[0017] Add 4-(4-aminophenyl)morpholin-3-one (192.2g, 1mol), 2-(2-chloroethoxy)propane (203.2g, 1mol) and 790ml ethanol to a 1000ml three-necked flask, and start Stir, heat up to reflux, point TLC detection, it is found that the raw material disappears after 10 hours of reaction, the reaction is completed, and the temperature is lowered to room temperature. Suction filtration, washing with 100 ml of ethanol three times, and drying to obtain 363.78 g of the compound (4) with a yield of 92% and a detection rate of 98.67% by HPLC.
[0018] 1 HNMR (600MHz, DMSO-d6, δppm): 7.90-7.76 (m, 4H), 7.04 (d, 2H), 6.62 (d, 2H), 5.67 (t, 1H), 5.20 (d, 1H), 4.20 ( s, 2H), 4.10-3.85 (m, 3H), 3.71-3.55 (m, 4H), 3.28-2.90 (m, 2H);
Embodiment 2
[0020] Preparation of (S)-4-(4-((3-amino-2-hydroxypropyl)aminophenyl)morpholin-3-one hydrochloride (5)
[0021]
[0022] Add 2-[(2R)-2-hydroxyl-3-[[4-(3-oxo-4-morpholine)phenyl]amino]propyl]-1H-isoindole-1 into a 2000ml three-necked flask , 3(2H)-diketone (360g, 0.910mol), 30% methylamine solution 377g and 1440ml ethanol were stirred, the temperature was raised to 65°C, and the reaction was carried out for 8h, and the reaction was detected by TLC. Add 25% HCl solution to adjust pH=4-5. Rotary evaporate to dryness, add 700ml of ethanol to beat at room temperature for 2 hours, filter with suction, wash with 200ml of ethanol three times, and dry the compound (5) in a total of 242.37g with a yield of 88.26%. HPLC detection 99.24%.
[0023] 1 HNMR (600MHz, DMSO-d6, δppm): 7.60 (d, 2H), 7.42 (d, 2H), 5.69 (t, 1H), 5.24 (d, 1H), 4.68-4.59 (m, 1H), 4.20 ( s, 2H), 4.18(t, 1H), 3.95(d, 2H), 3.89(d, 1H), 3.75(d, 2H), 2.94-2.78(m, 2H), 1.77-1.65(s, 2H) ;
Embodiment 3
[0025] (S)-5-chloro-N-(3-(5-chlorothiophene-2-formylamino)-2-hydroxypropyl)-N-(4-(3-oxomorpholino)phenyl) Preparation of thiophene-2-carboxamide
[0026]
[0027] Add (S)-4-(4-((3-amino-2-hydroxypropyl)aminophenyl)morpholin-3-one hydrochloride (5) (240g, 0.795mol) into a 3000ml three-necked flask, Stir triethylamine (321.78g, 3.18mol), dichloromethane 1400ml, stir in an ice bath, weigh 5-chloro-2-acylchlorothiophene (359g, 1.99mol) solution in 600ml dichloromethane, add dropwise to the reaction flask , stirred in an ice bath for 2 hours, reacted at room temperature for 4 hours, and the reaction was detected by TLC. Added 600ml of water, stirred to have solid precipitation, suction filtered, washed three times with 100ml of dichloromethane, dried to obtain 377.46g, yield 85.63%, HPLC detected 98.95% .
[0028] 1 HNMR (600MHz, DMSO-d6, δppm): 8.96(t, 1H), 8.64(t, 1H), 7.85(d, 1H), 7.74(d, 1H), 7.56(d, 2H), 7.42(d, 2H), 7.21(d, 1H), 5.55(d, 1H), 4.95-4.77(m, 1H), 4.25-4.14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com