New application of paeoniflorin
A technology of paeoniflorin and drugs, applied in the field of medicine, can solve the problems of long course of treatment, high cost, and ineffective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
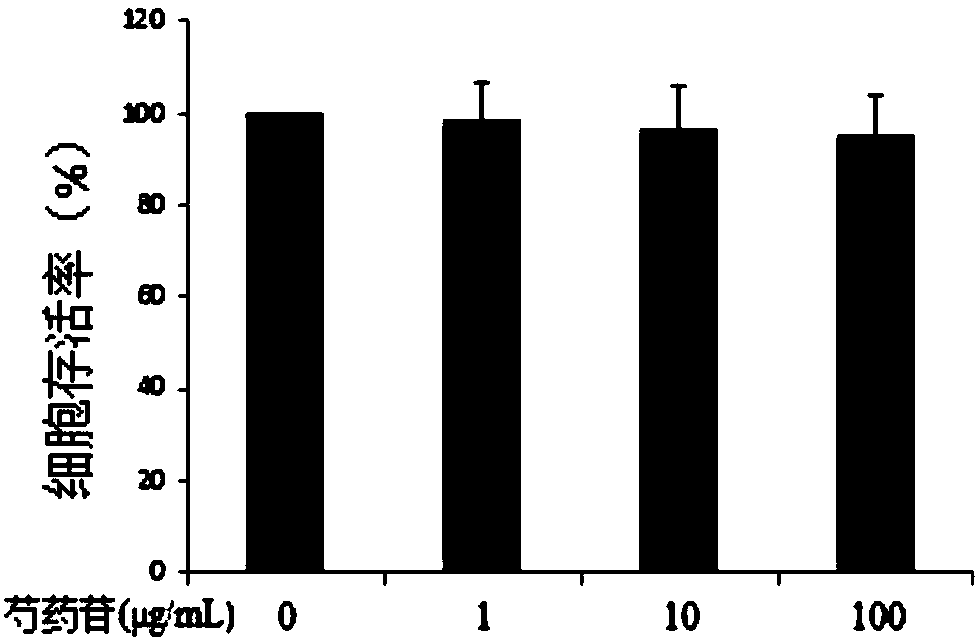
[0019] Experimental purpose and method: No matter what kind of drug, if the concentration is too high, it will have cytotoxic effect on the cells. Too high concentration of paeoniflorin will cause certain toxicity to P815 cells. In order to detect the toxicity of paeoniflorin to P815, CCK- 8 Experiments were performed to test the cell survival rate of different concentrations of paeoniflorin after stimulating P815 for 24 hours to determine the concentration of paeoniflorin that has no growth inhibitory activity on the cells. The specific steps are as follows:
[0020] (1) Cultivation and passage of mast cell line P815
[0021] A. Cultivation of P815 cells: P815 was cultured in high glucose DMEM medium, containing 10% fetal bovine serum (FBS), 1% penicillin, cultured at 37°C, 5% CO 2 Environment;
[0022] B. Passaging of P815 cells: When the cell density in step A reaches 70%, passaging is required. Collect the cell culture fluid in the culture flask in a centrifuge tube, rinse three ...
Embodiment 2
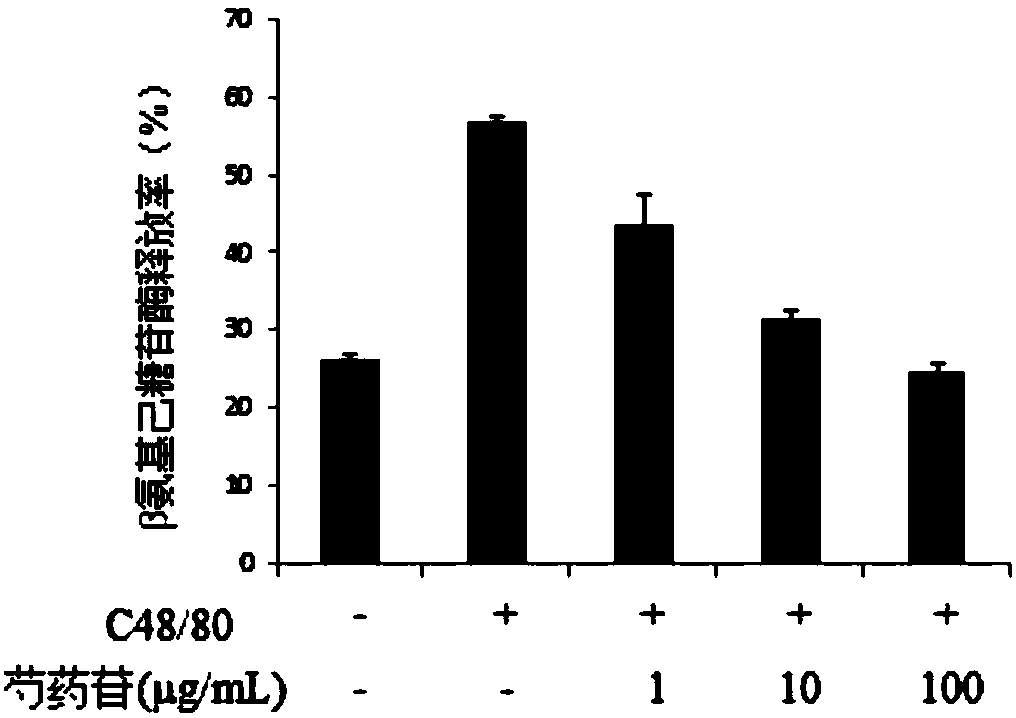
[0030] Experimental purpose and method: In order to verify whether paeoniflorin can inhibit mast cell degranulation, mast cells were pretreated with different concentrations of paeoniflorin to detect whether it can inhibit the release of mast cell degranulation marker β-hexosaminidase. Specific steps as follows:
[0031] (1) Cultivation and passage of mast cell line P815
[0032] A. Cultivation of P815 cells: P815 was cultured in high glucose DMEM medium, containing 10% fetal bovine serum (FBS), 1% penicillin, cultured at 37°C, 5% CO 2 Environment;
[0033] B. Passaging of P815 cells: When the cell density in step A reaches 80%, passaging is required. Collect the cell culture fluid in the culture flask in a centrifuge tube, rinse three times with preheated phosphate buffered saline solution, and add appropriate amount of trypsin. After the cells are completely digested, add the cell culture medium to neutralize the trypsin, pipette evenly, centrifuge at 800 rpm for 3 minutes, remove...
Embodiment 3
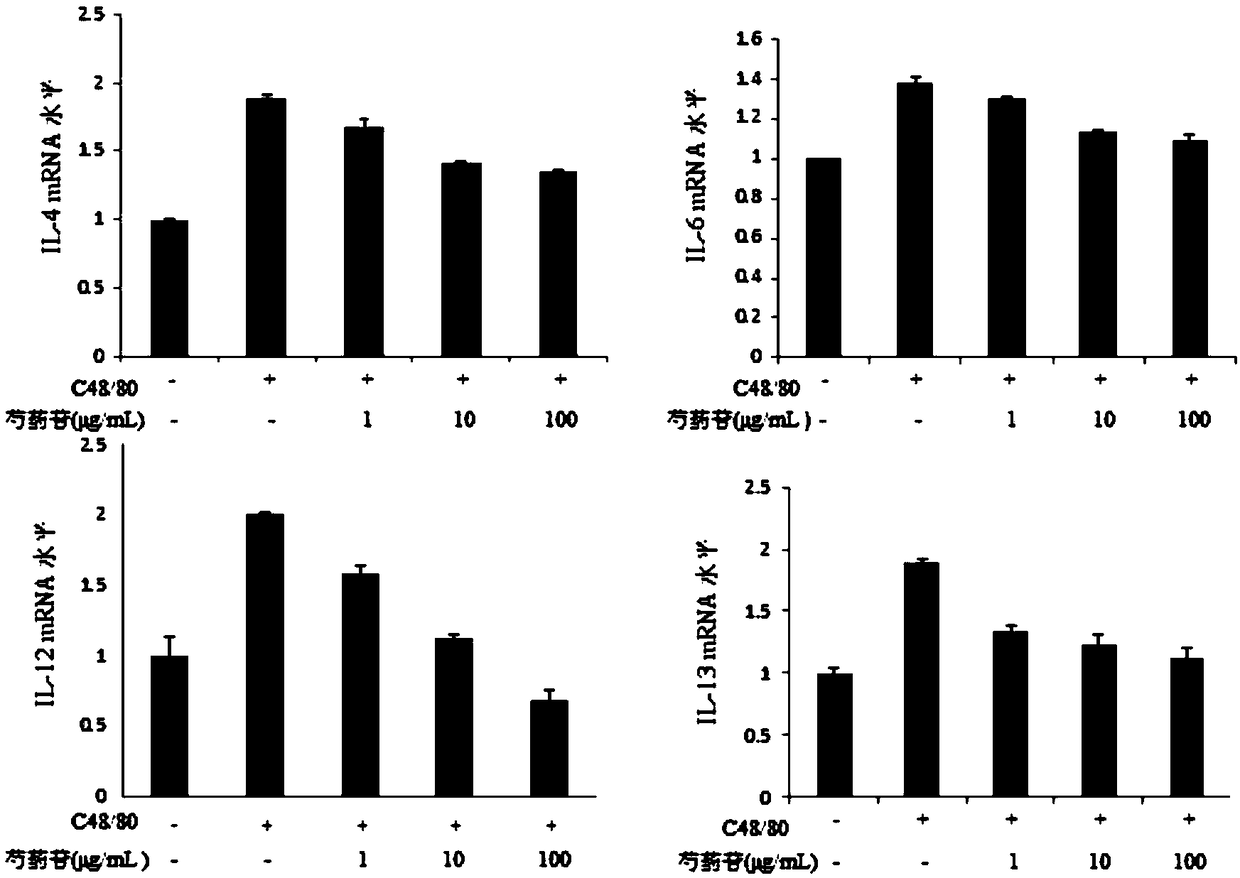
[0041] Experimental purpose and method: To detect the effect of paeoniflorin on the expression of IL-4, IL-6, IL-12, IL-13, PAR2, PAR3, PAR4 at the mRNA level after P815 activation, the specific steps are as follows:
[0042] (1) Cultivation and passage of mast cell line P815
[0043] A. Cultivation of P815 cells: P815 was cultured in high glucose DMEM medium, containing 10% fetal bovine serum, 1% penicillin, and cultured at 37°C, 5% CO 2 Environment;
[0044] B. Passaging of P815 cells: When the cell density in step A reaches 70%, passaging is required. Collect the cell culture fluid in the culture flask in a centrifuge tube, rinse three times with preheated phosphate buffered saline solution, and add appropriate amount of trypsin. After the cells are completely digested, add the cell culture medium to neutralize the trypsin, pipette evenly, centrifuge at 800 rpm for 3 minutes, remove the supernatant, add fresh high-sugar DMEM medium and pipette to mix the cells, and pass the cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com