Polycaprolactone-base diethylsulfopropyl betaine, preparation method thereof, and construction method with same as medicine release carrier
A technology of polycaprolactone-based diethylsulfopropyl beet and caprolactone-based diethylsulfopropyl beet, which is applied in the field of biomedical polymer materials, can solve problems such as poor long-term circulation ability, and achieve simple operation , good anti-protein adsorption performance, prolonging the effect of cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: polycaprolactone base diethylsulfopropyl betaine preparation (I)
[0031] (1) Take by weighing 1.17g diethylethanolamine and be dissolved in 50ml toluene, magnetic stirring is mixed with the diethylethanolamine solution that mass concentration is 2.6%;
[0032] (2) Weighing 1.2g propane sultone was added and dissolved in 50ml toluene, and magnetically stirred to form a propane sultone solution with a mass concentration of 2.7%;
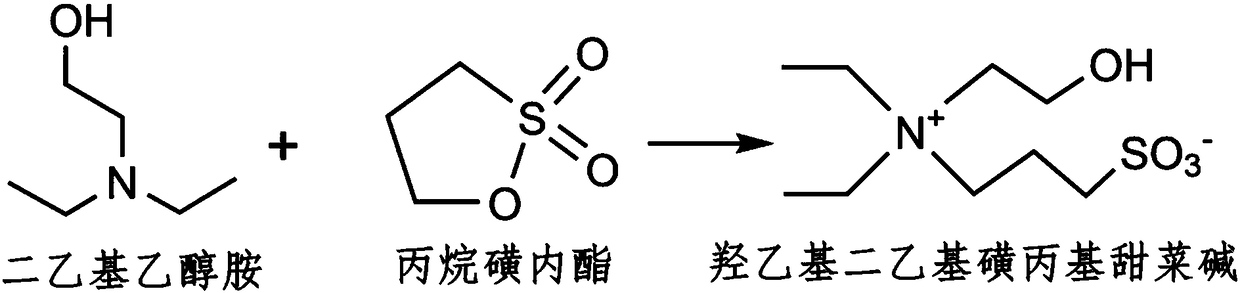
[0033] (3) Add the propane sultone solution obtained in step (2) dropwise to the diethylethanolamine solution obtained in step (1) at 50° C. and nitrogen gas, at a rate of 3 ml / min. After continuing to react for 12h, stop the reaction, filter the product and recrystallize in isopropanol to obtain hydroxyethyldiethylsulfopropyl betaine (SB), the reaction process is as follows figure 1 shown;
[0034] (4) 1.34g caprolactone and 0.22g SB are dissolved in 50ml toluene solution, wherein the mass concentration of caprolactone is 3%, t...
Embodiment 2
[0036] Embodiment 2: preparation of polycaprolactone-based diethylsulfopropyl betaine (II)
[0037] (1) Take by weighing 11.7g diethylethanolamine and be dissolved in 50ml toluene, magnetic stirring is mixed with the diethylethanolamine solution that mass concentration is 21.27%;
[0038] (2) Weighing 12.2g propane sultone was added and dissolved in 50ml toluene, and magnetically stirred to form a propane sultone solution with a mass concentration of 22%;
[0039] (3) Add the propane sultone solution obtained in step (2) dropwise to the diethylethanolamine solution obtained in step (1) at 60° C. and nitrogen gas, at a rate of 6 ml / min. After continuing to react for 24 hours, stop the reaction, filter the product and recrystallize in isopropanol to obtain hydroxyethyldiethylsulfopropyl betaine (SB);
[0040](4) 50.81g caprolactone and 2.27g SB are dissolved in 50ml toluene solution, wherein the mass concentration of caprolactone is 53%, and the concentration of SB is 2.5%; Aft...
Embodiment 3
[0042] Embodiment 3: preparation of polycaprolactone-based diethylsulfopropyl betaine (III)
[0043] (1) Take by weighing 23.4g diethylethanolamine and be dissolved in 50ml toluene, magnetic stirring is mixed with the diethylethanolamine solution that mass concentration is 35%;
[0044] (2) Weighing 24.4g propane sultone was added and dissolved in 50ml toluene, and magnetically stirred to form a propane sultone solution with a mass concentration of 35%;
[0045] (3) Add the propane sultone solution obtained in step (2) dropwise to the diethylethanolamine solution obtained in step (1) at 60° C. and nitrogen gas, at a rate of 8 ml / min. After continuing to react for 36 hours, stop the reaction, filter the product and recrystallize in isopropanol to obtain hydroxyethyldiethylsulfopropyl betaine (SB);
[0046] (4) 130.5g caprolactone and 0.48g SB are dissolved in 50ml toluene solution, wherein mass concentration of caprolactone is 75%, the concentration of SB is 0.2%; After mixing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com