Pharmaceutical composition, anti-tumor pharmaceutical containing same
An anti-tumor drug and composition technology, applied in the field of pharmacy, can solve the problems of high toxicity, difficult to further improve the therapeutic efficiency, easy to induce multidrug resistance, etc., and achieve the effect of wide application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
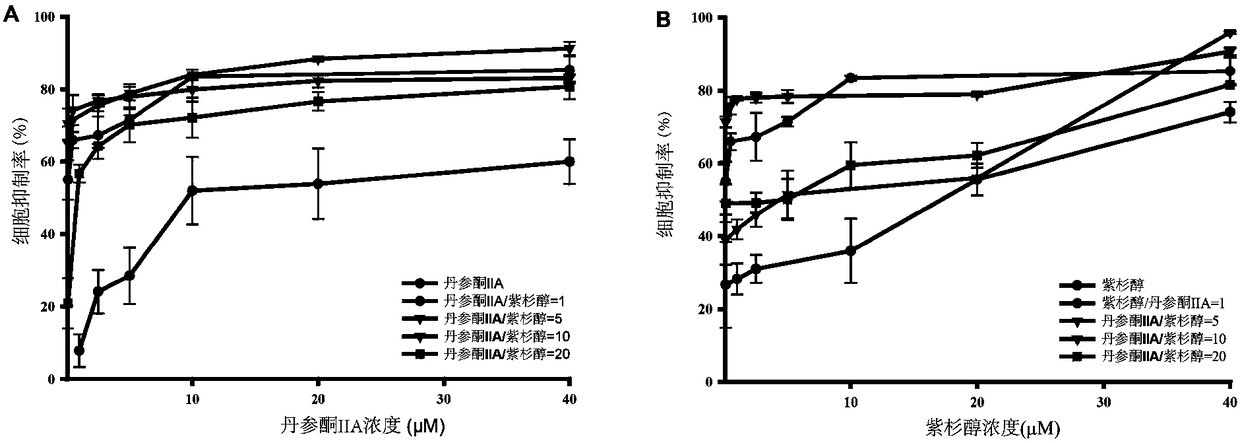
[0041] This test selects tanshinone Ⅱ A and paclitaxel in the compound concentration range of the pharmaceutical composition described in the present invention to NB 4 Cells (acute promyelocytic leukemia cells) were tested for anti-tumor activity, and the attenuating and synergistic effects of tanshinone IIA and paclitaxel at the compound concentration described in the present invention were verified by comparing with the individual concentration of tanshinone IIA and paclitaxel.
[0042] 1. Test material:
[0043] (1) Cultivation of test cell lines:
[0044] will NB 4 Cells (purchased from Jiangsu KGI Biotechnology Co., Ltd.) were inoculated on RPMI 1640 complete medium in 5% CO 2 , 37°C and saturated humidity conditions, replace the medium every 2 to 3 days, and take the NB in the logarithmic growth phase 4 Cells are tested.
[0045] The RPMI 1640 complete medium contains 10% newborn bovine serum, 100 IU / mL penicillin and 100 μg / L streptomycin.
[0046] (2) Preparatio...
Embodiment 2
[0070] In this test, Tanshinone IIA and paclitaxel were selected to detect the anti-tumor activity of Hepg-2 cells (human liver cancer cells) within the compound concentration range of the pharmaceutical composition described in the present invention. Attenuation and synergism of tanshinone Ⅱ A and paclitaxel at the compound concentration of the present invention.
[0071] 1. Test material:
[0072] (1) Cultivation of test cell lines:
[0073] Hepg-2 cells (from Shanghai University of Traditional Chinese Medicine Teaching and Experimental Center Laboratory) were inoculated on RPMI1640 complete medium in 5% CO 2 , 37°C and saturated humidity conditions, the medium was replaced every 2-3 days, and the Hepg-2 cells in the logarithmic growth phase were used for experiments.
[0074] The RPMI 1640 complete medium contains 10% newborn bovine serum, 100 IU / mL penicillin and 100 μg / L streptomycin.
[0075] (2) Preparation of drug mother solution:
[0076] Tanshinone Ⅱ A powder (pu...
Embodiment 3
[0098] The preparation of embodiment 3 pharmaceutical compositions
[0099] Take the tanshinone IIA raw material drug and prepare a tanshinone IIA solution with a final concentration of 1.5 μM with chloroform; take the paclitaxel raw material drug and prepare a paclitaxel solution with a final concentration of 0.3 μM with chloroform; mix the above-mentioned tanshinone IIA solution with the paclitaxel solution to obtain a drug combination (the ratio of paclitaxel and tanshinone IIA is 1:5)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com