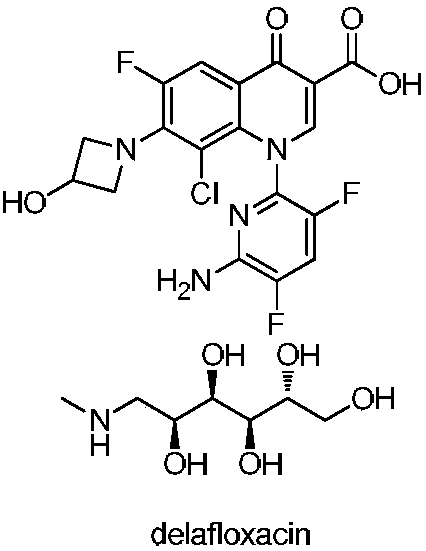
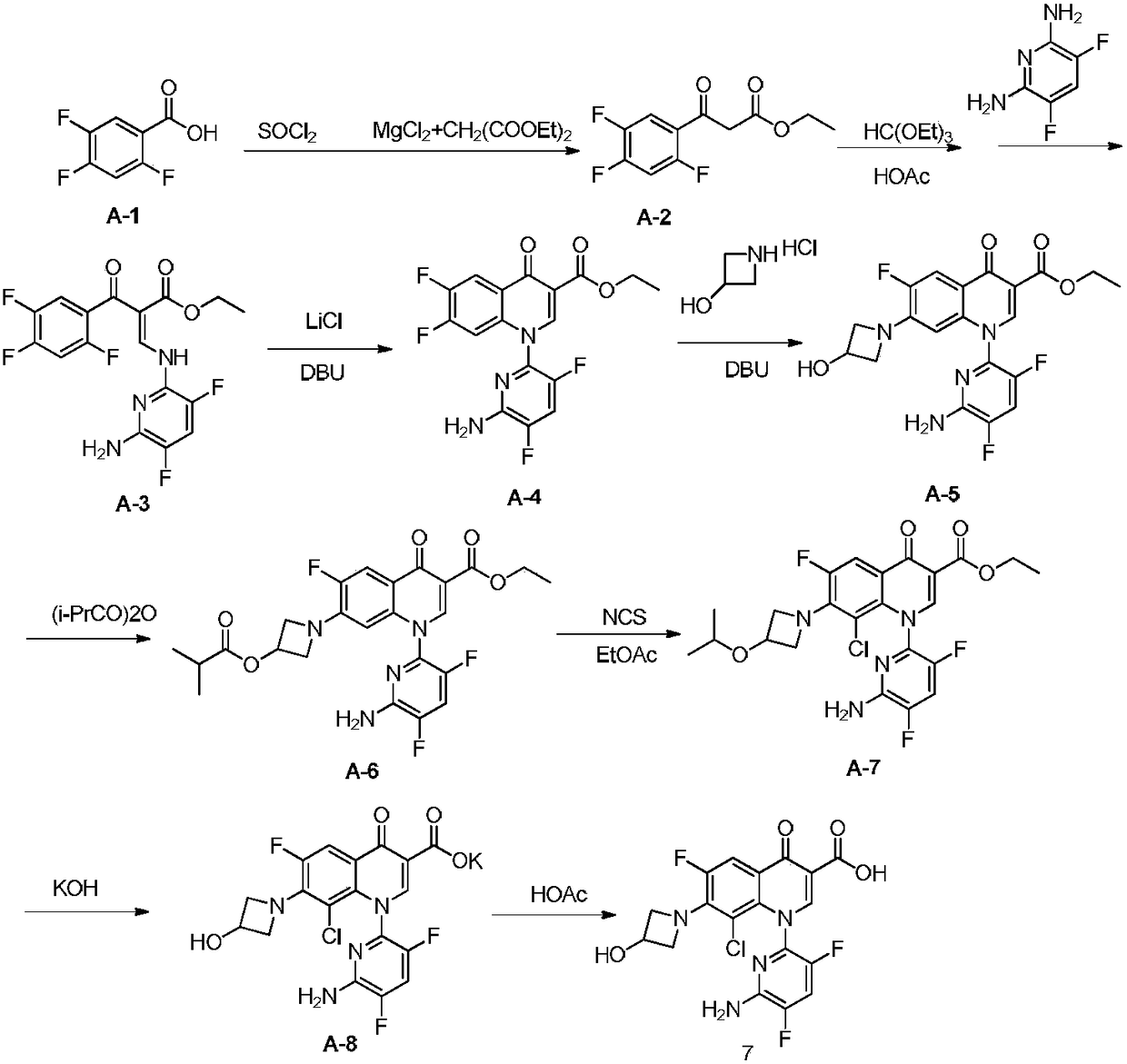
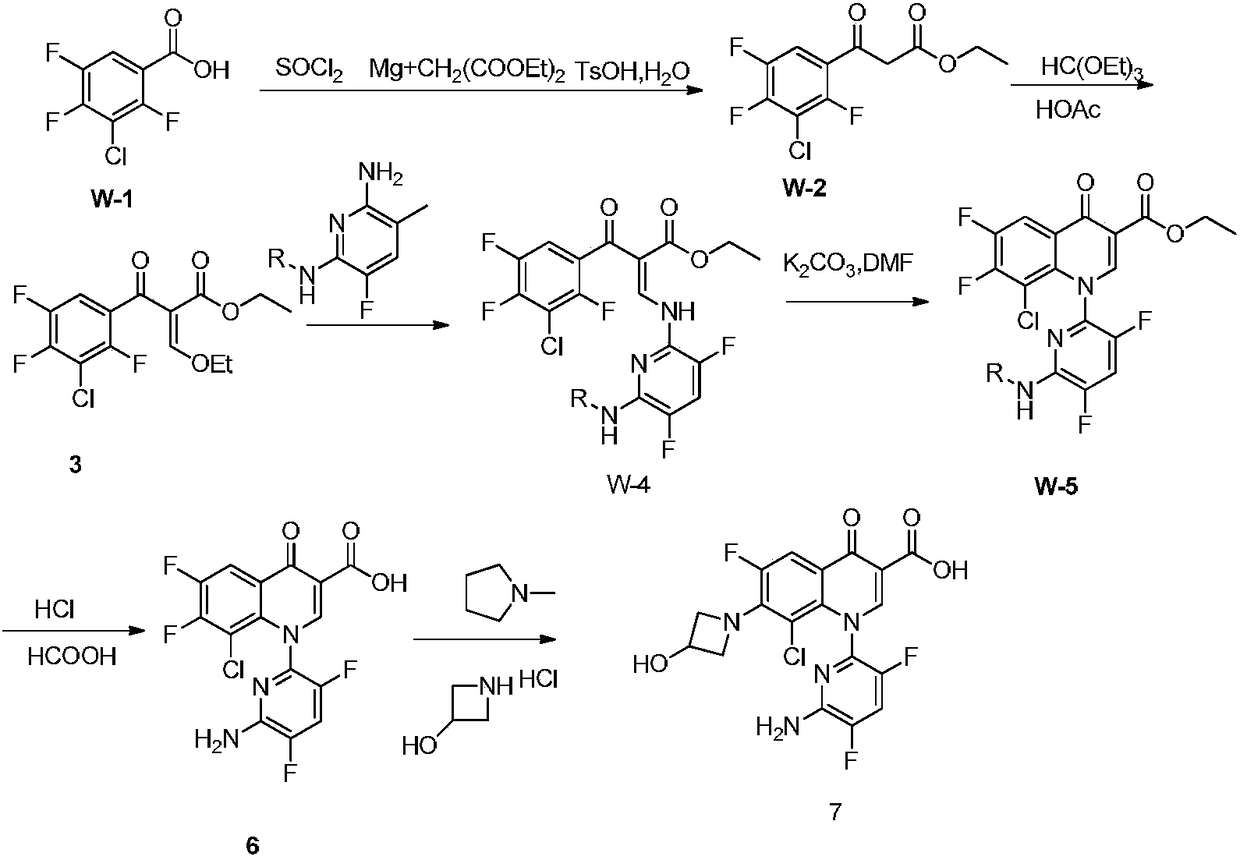
Preparation method of Delafloxacin and intermediates thereof
A technology of delafloxacin and intermediates, applied in the field of medicine, can solve the problems of amidation reaction, high reaction temperature, multiple impurities and the like, and achieve the effects of simple operation, high purity and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of 3-chloro-2,4,5-fluorobenzoyl chloride (compound 2)
[0043] (1) Compound 1 (5g, 23.7mmol) and dichloromethane (50ml) were mixed and stirred, and thionyl chloride (5.6g, 44.4mmol) was added dropwise at 0-20°C. After the addition was complete, DMF (0.2ml ), heated to reflux, monitored by TLC, it took 8 hours to complete the reaction, cooled, and the solvent was removed under reduced pressure to obtain 5.4 g of compound with a yield of 99.5%.
Embodiment 2
[0045] Preparation of ethyl 2-(3-chloro-2,4,5-trifluorobenzoyl)-3-(dimethylamino)acrylate (compound 3)
[0046] (1) Mix N,N-ethyl dimethylaminoacrylate (4.2g, 32.7mmol), triethylamine (2.6g, 26.2mmol), dichloromethane (40ml), drop compound 1 (5g, 21.8mmol ) dichloromethane (20ml) mixed solution, heated to 1, TLC monitoring, need 3 hours to complete the reaction, concentrated to give compound 3 7.3g yield 99%.
Embodiment 3
[0048] Preparation of ethyl 3-((6-amino-3,5-difluoro-2-pyridyl)amino)-2-(2,4,5-trifluorobenzoyl)acrylate (compound 4)
[0049] (1) Compound 3 (7g, 20.8mmol); acetonitrile (70ml), glacial acetic acid (3ml) was mixed, and 2,4-diamino-3,5-difluoropyridine (3.3g, 22.8 mmol) of acetonitrile solution, heated to 30-40°C after the dropwise addition, monitored by TLC, it took 1 hour to complete the reaction, cooled, slowly added an appropriate amount of water to the reaction solution, stirred, filtered, and dried to obtain 8.6g of compound 4 with a yield of 94.9% . 1H NMR (400MHz, CDCl3) δ11.31(d,1H),9.04(d,1H),7.41(td,1H),7.25(t,1H),4.65(m,2H),4.17(q,2H) ,1.11(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com