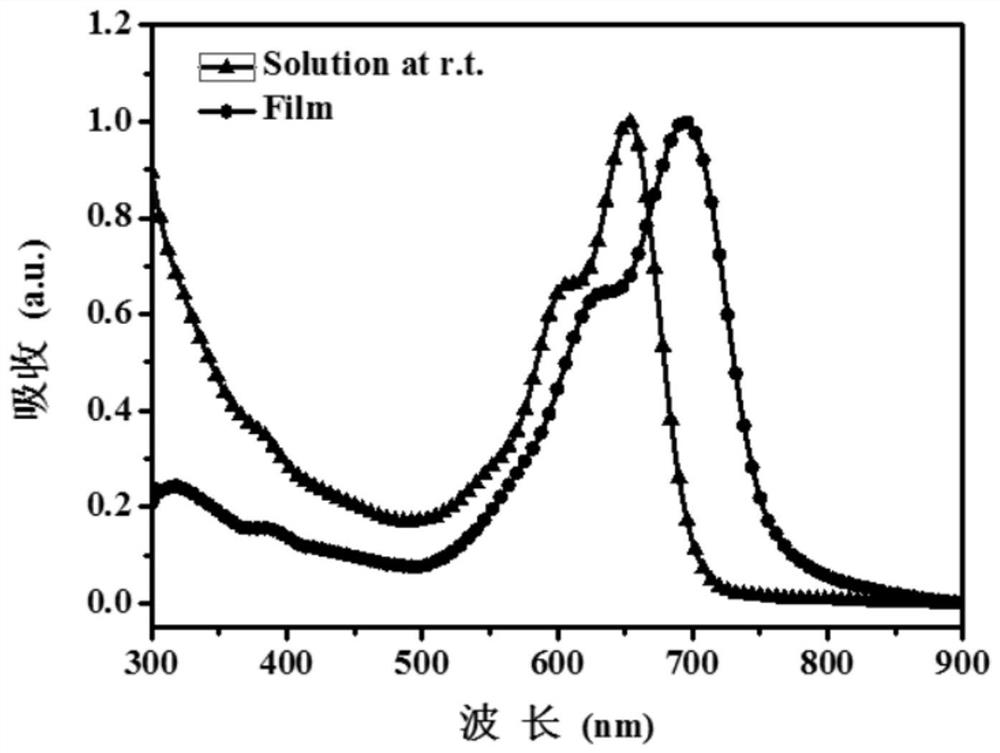
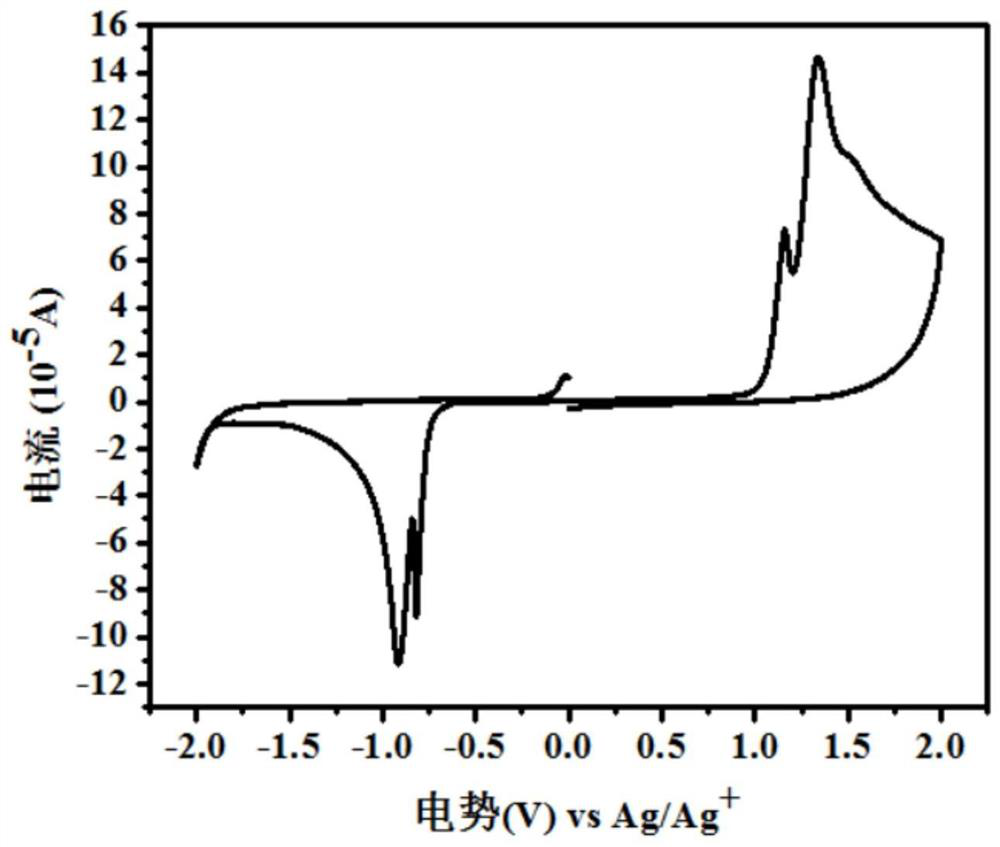
A kind of asymmetric cyanomethylene indoketone acceptor material and its preparation method and application
A cyanomethylene indone, asymmetric technology, applied in the field of organic solar cells, can solve the problems of difficult energy level regulation, high synthesis cost, weak absorption, etc., and achieve high-efficiency charge transfer, excellent device performance, and good device performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
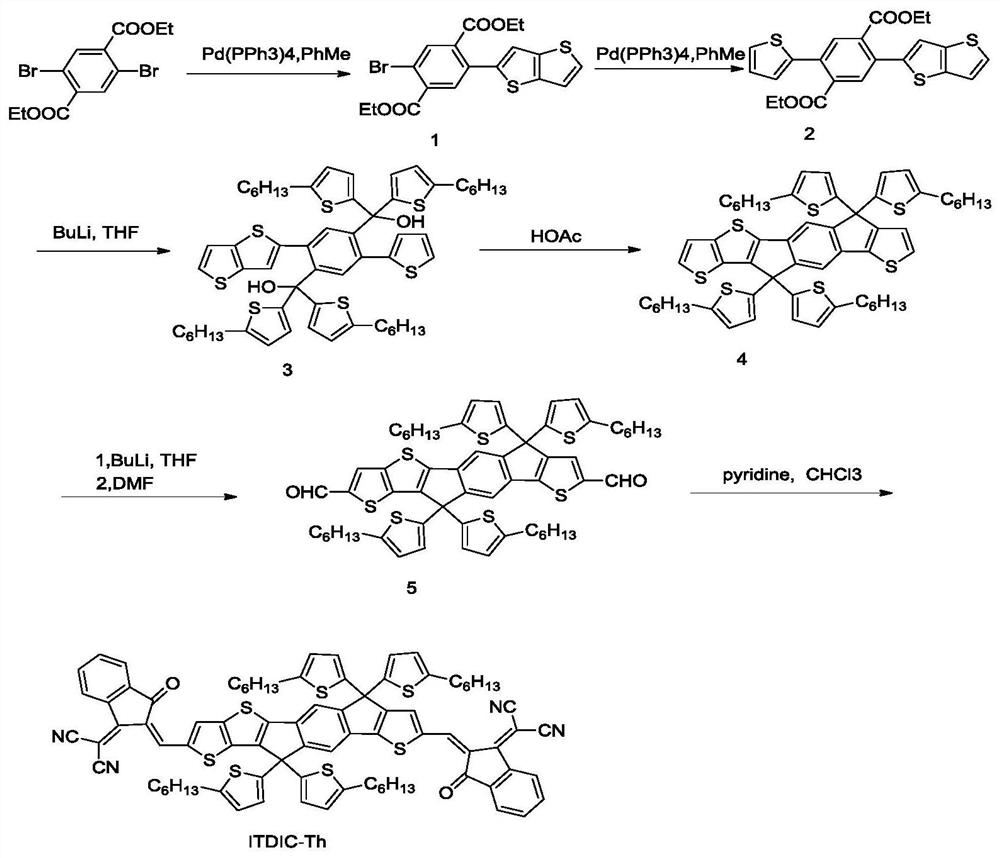
[0035] The preparation method of asymmetric cyanomethylene indoketone electron acceptor material ITDIC-Th, the specific steps are as follows:
[0036] (1) Synthesis of Compound 1: Diethyl 2,5-dibromobenzoate (10mmol, 3.8g), thiophenostinane (1equiv, 10mmol, 4.3g) and tetrakistriphenylphosphine palladium (0.12mmol, 140mg) into the dried 250ml double-necked bottle, N 2 For protection, add about 50ml of anhydrous toluene; heat and reflux overnight, spin off the toluene directly after the reaction solution is cooled, add about 60ml of petroleum ether for washing and suction filtration, and use petroleum ether: ethyl acetate = 10:1 column chromatography for the filtrate to obtain yellow Solid, namely compound 1 (3mmol, 1.318g, 30%, 1 HNMR (500MHz, CDCl 3 ( m, 2H), 1.426(t, J=14Hz, 3H), 1.140(t, J=14.5Hz, 3H)).
[0037](2) Synthesis of compound 2: compound 1 (3mmol, 1.318g), thiophenestannane (1.2equiv, 3.5mmol, 1.306g) and tetrakistriphenylphosphine palladium (0.06mmol, 70mg) w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com