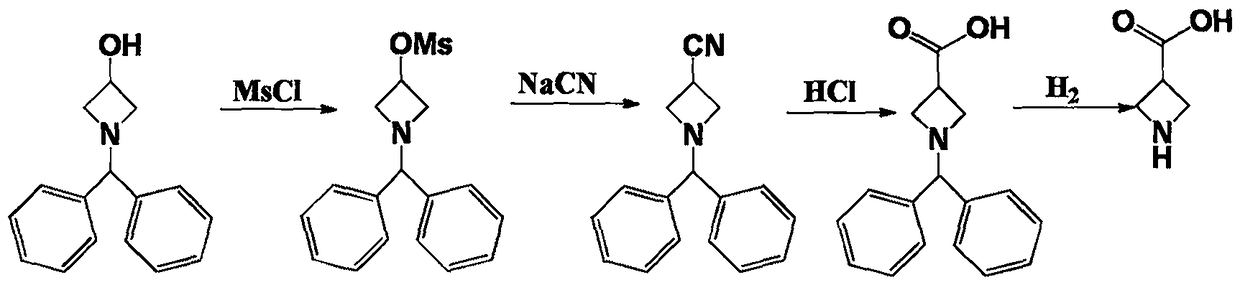
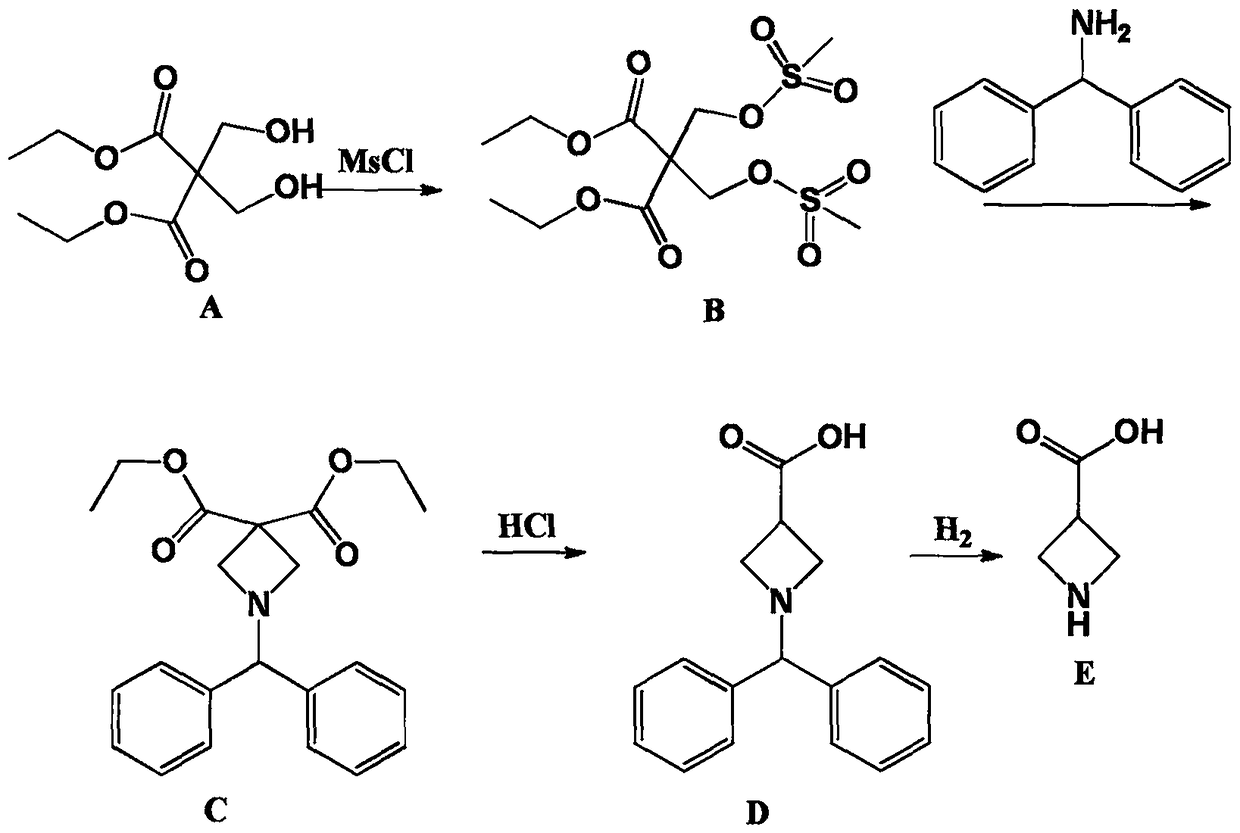
Synthetic method of azetidine-3-formic acid
A technology of azetidine and synthesis method, applied in the direction of organic chemistry and the like, can solve the problem that the price of downstream products remains high, the poisoning of operators, and the high market price of diphenylmethyl-3-hydroxy azetidine. and other problems, to achieve the effect of large-scale industrial production prospects, cost reduction, avoidance of human injury and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthesis of intermediate product (B)
[0024] Add 110g of 2,2-dimethylolmalonate-1,3-diethyl ester, 1.5L of dichloromethane, and 150g of triethylamine into a clean 3L three-necked flask, stir mechanically for 10min, cool down to 0°C, and slowly Add 115g of methanesulfonyl chloride dropwise, control the temperature at 0-5°C, a large amount of solid salts are formed, react overnight at room temperature after dropping, add 1L of water to wash the next day, separate the layers, dry the organic layer, concentrate and dry to obtain the intermediate product (B) 180g, yield 95.7%, without purification.
Embodiment 2
[0025] Embodiment 2: the synthesis of intermediate product (C)
[0026] Add 1.5L of toluene to a clean 3L three-necked flask, then add 180g of the intermediate product (B) obtained in Example 1, continue to add 87g of diphenylmethylamine and 150g of triethylamine, heat to 110°C for reflux reaction, and a solid is formed , refluxed overnight, stopped the reaction, filtered to remove salt, washed the organic layer with water, dried and concentrated to obtain 120 g of intermediate product (C), which did not need to be purified.
Embodiment 3
[0027] Embodiment 3: the synthesis of intermediate product (D)
[0028] Add 120g of the intermediate product (C) obtained in Example 2 into 1L of 6N hydrochloric acid, heat to 80°C, keep it warm overnight, and gas is generated, pay attention to prevent flushing, and evaporate the water to dryness the next day to obtain a brown oily object , adding 300mL of acetone and heating for recrystallization to obtain 78g of intermediate product (D), the appearance of which is light yellow solid. 1H-NMR Spectrum (CDCl3), δ (ppm): 3.00-3.90 (5H, m), 4.95 (1H, s), 7.25-7.28 (2H, m), 7.33 (4H, m), 7.53 (4H, m ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com