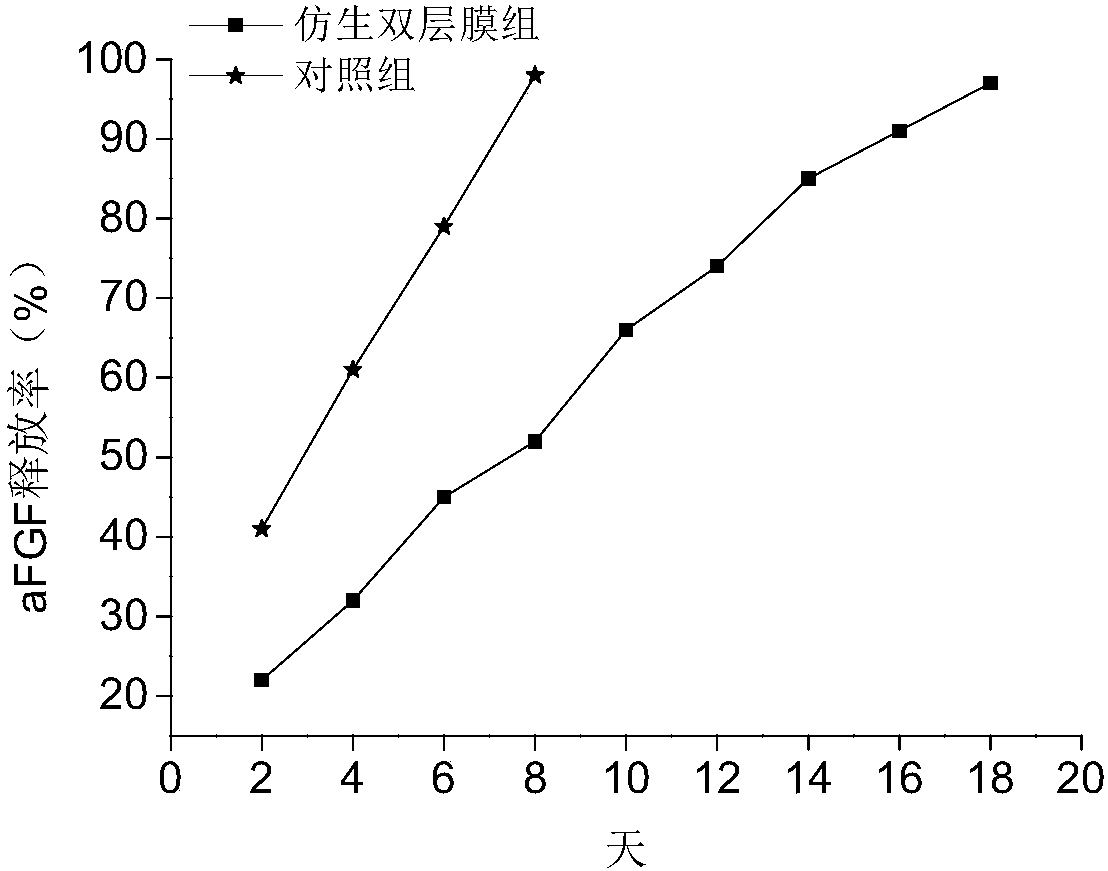
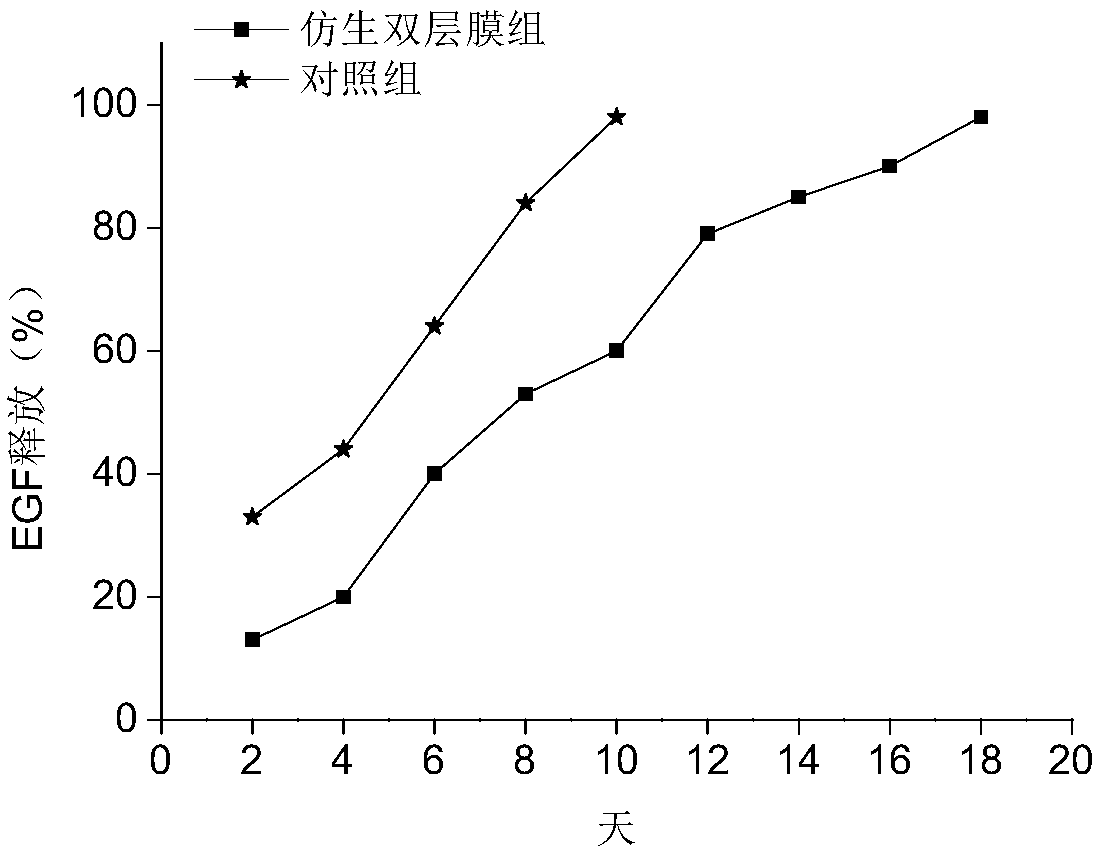
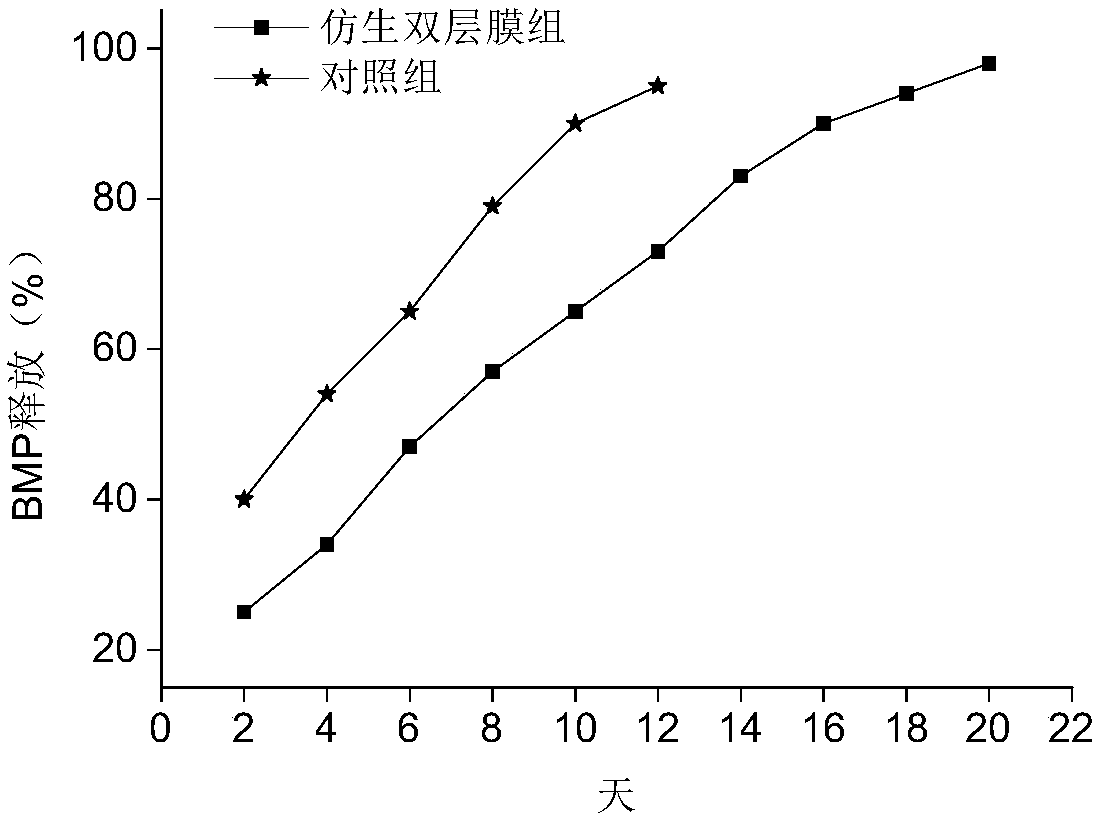
Bionic double-layer sustained-release growth factor film as well as preparation method and application thereof
A growth factor, double-layer technology, applied in the field of regenerative medicine, can solve the problems of inactivation, decrease of growth factor activity, maintenance of effective concentration, etc., and achieve good binding and protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] First prepare the gelatin containing 3% (w / v, g / ml) alkaline gelatin (jelly strength 150Bloom g), 10% (w / v, g / ml) chitosan (molecular weight 100000kDa), 0.1% (w / v , g / ml) hyaluronic acid (molecular weight 3,000,000 kDa), 10% (v / v) glycerol, 0.2% (w / v, g / ml) sodium carboxymethyl cellulose gel-forming aqueous solution of the positive layer. The positive layer colloidal aqueous solution and formaldehyde aqueous solution are mixed evenly, and the final concentration of formaldehyde is 3% (w / v, g / ml). Then spread the mixed solution on the polytetrafluoroethylene template to carry out the cross-linking reaction. The reaction conditions are temperature 22°C, humidity 20%, and reaction time 6 hours to obtain a positive charge layer. Then, prepare 15% (w / v, g / ml) acid gelatin (jelly strength 150Bloom g), 1% (w / v, g / ml) chitosan (molecular weight 300000kDa), 1% (w / v , g / ml) hyaluronic acid (molecular weight 1000000kDa), 6% (v / v) glycerol, 2% (w / v, g / ml) sodium carboxymethyl cell...
Embodiment 2
[0048] First prepare the gelatin containing 15% (w / v, g / ml) alkaline gelatin (jelly strength 220Bloom g), 1% (w / v, g / ml) chitosan (molecular weight 300000kDa), 1% (w / v , g / ml) hyaluronic acid (molecular weight 1000000kDa), 1% (v / v) glycerin, 2% (w / v, g / ml) sodium carboxymethyl cellulose gel-forming aqueous solution of positive charge layer. The positive layer gelling aqueous solution and the glutaraldehyde aqueous solution are mixed evenly, and the final concentration of glutaraldehyde is 0.01% (w / v, g / ml). Then spread the mixed solution on the polytetrafluoroethylene template to carry out cross-linking reaction. The reaction conditions are temperature 20° C., humidity 20%, and reaction time 8 hours to obtain a positive charge layer. Then, prepare 3% (w / v, g / ml) acid gelatin (jelly strength 250Bloom g), 10% (w / v, g / ml) chitosan (molecular weight 40000kDa), 0.1% (w / v , g / ml) hyaluronic acid (molecular weight 2000000kDa), 5% (v / v) glycerol, 0.7% (w / v, g / ml) sodium carboxymethyl...
Embodiment 3
[0050] First prepare the gelatin containing 15% (w / v, g / ml) alkaline gelatin (jelly strength 220Bloom g), 1% (w / v, g / ml) chitosan (molecular weight 300000kDa), 1% (w / v , g / ml) hyaluronic acid (molecular weight 1000000kDa), 1% (v / v) glycerin, 2% (w / v, g / ml) sodium carboxymethyl cellulose gel-forming aqueous solution of positive charge layer. The positive layer gelling aqueous solution and the glutaraldehyde aqueous solution are mixed evenly, and the final concentration of glutaraldehyde is 0.01% (w / v, g / ml). Then spread the mixed solution on the polytetrafluoroethylene template to carry out cross-linking reaction. The reaction conditions are temperature 20° C., humidity 20%, and reaction time 8 hours to obtain a positive charge layer. Then, prepare 15% (w / v, g / ml) acid gelatin (jelly strength 250Bloom g), 1% (w / v, g / ml) chitosan (molecular weight 300000kDa), 1% (w / v , g / ml) hyaluronic acid (molecular weight 1000000kDa), 1% (v / v) glycerin, 2% (w / v, g / ml) sodium carboxymethyl ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com