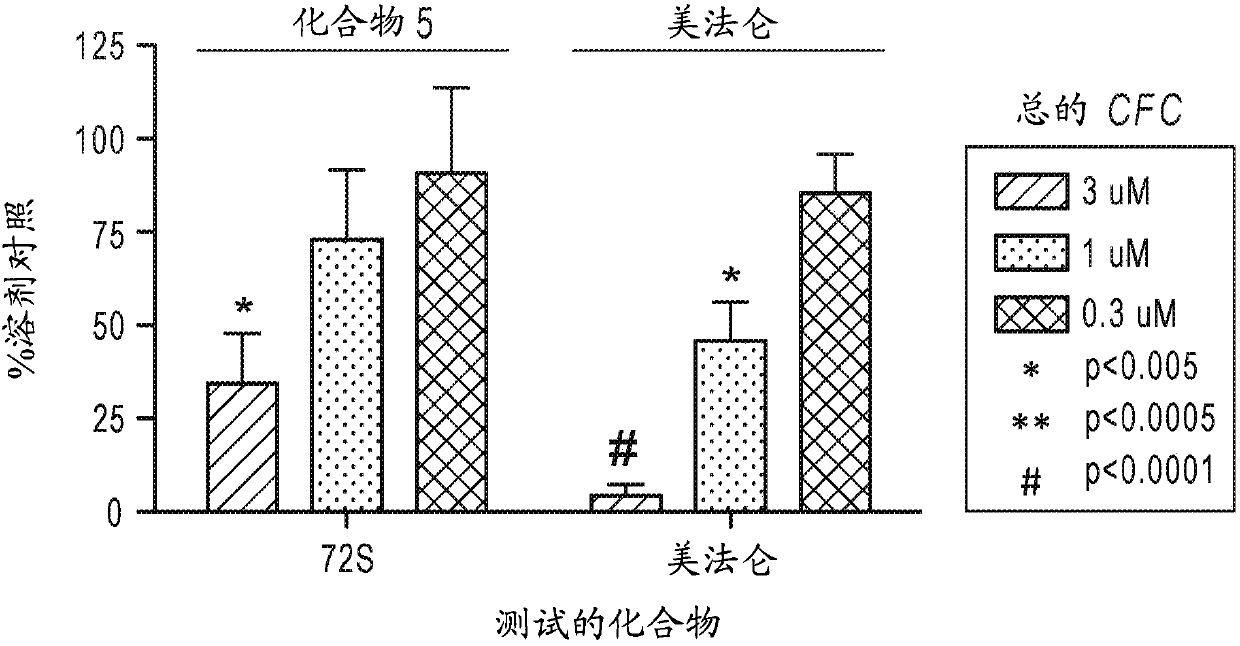
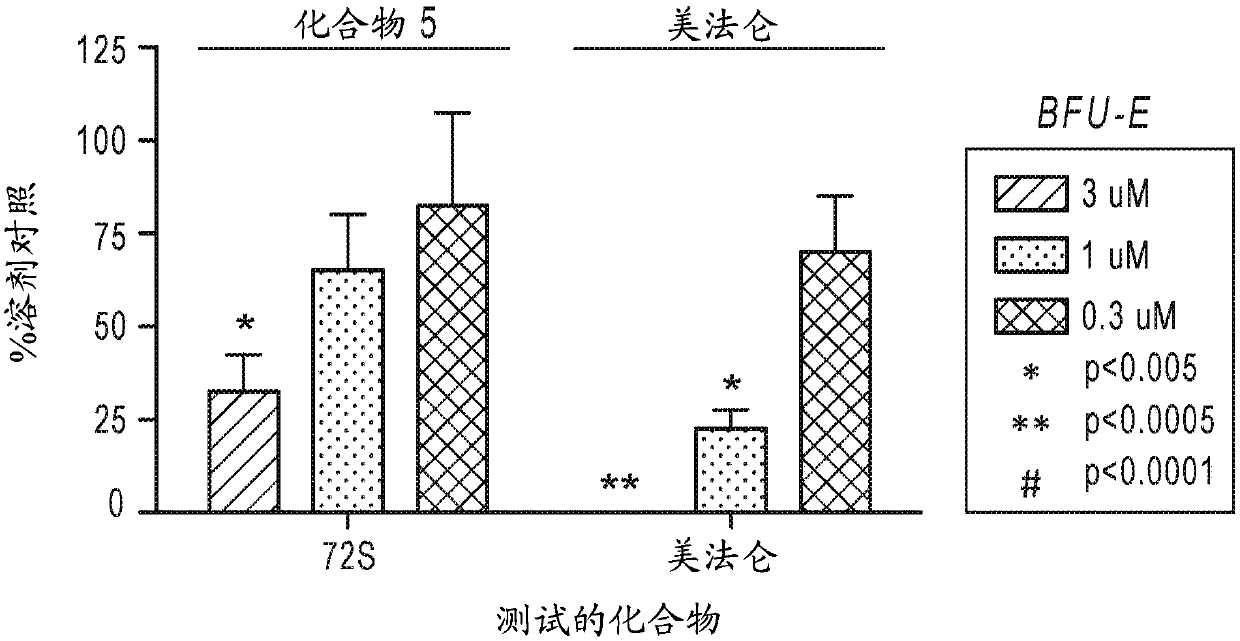
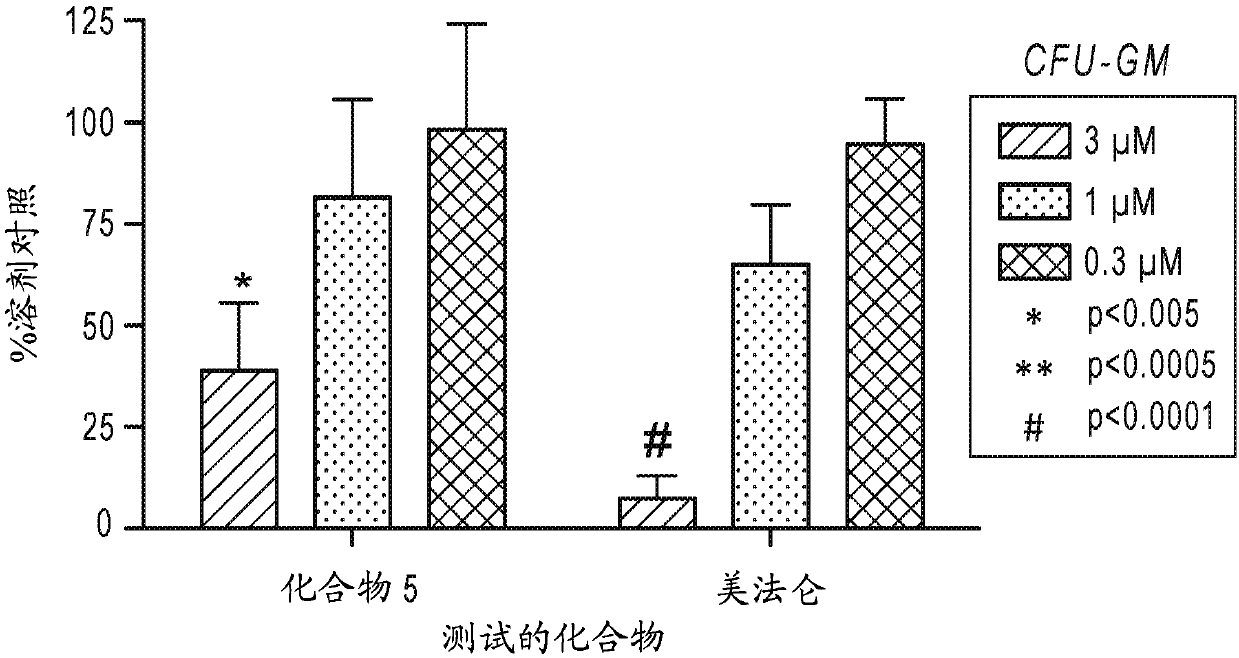
Beta-substituted beta-amino acids and analogs as chemotherapeutic agents and uses thereof
A compound and hydrocarbon-based technology, applied in the direction of chemical instruments and methods, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., can solve the problem of not giving the composition, etc., and achieve the effect of high intake selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1457] 3-Amino-3-[5-[bis(2-chloroethyl)amino]-2-methyl-phenyl]propanoic acid (1)
[1458]
[1459] Step A: (2-Methyl-5-nitro-phenyl)methanol (1a)
[1460] Following the general procedure described in Description 1, borane dimethyl sulfide complex (2.0M BH 3 ·SMe 2 (2-Methyl-5-nitro-phenyl)methanol (1a) was prepared from commercially available 2-methyl-5-nitrobenzoic acid (50.0 g, 276 mmol) (166 mL, 332 mmol) to give 44.0 g (˜quantitative yield) of the target compound (1a) as a pale yellow solid of sufficient purity to be used directly in the next step without further isolation and purification. R f : ~0.50 (EtOAc / Hxn=1:1, v / v). 1 H NMR (300MHz, CDCl 3 ): δ8.30(d, J=2.4Hz, 1H), 8.05(dd, J=8.4, 2.4Hz, 1H), 7.31(d, J=8.1Hz, 1H), 4.78(d, J=5.1Hz , 2H), 2.41 (s, 3H), 1.87 (br.t, J=5.1Hz, 1H) ppm. The compounds are also commercially available.
[1461] Step B: 2-Methyl-5-nitro-benzaldehyde (1b)
[1462] Following the general procedure described in 2 (Variant A), dimethyl...
Embodiment 2
[1478] 3-Amino-3-[4-[bis(2-chloroethyl)amino]-2-methyl-phenyl]propanoic acid (2)
[1479]
[1480] Step A: (2-Methyl-4-nitro-phenyl)methanol (2a)
[1481] Following the general procedure described in Description 1, borane dimethyl sulfide complex (2.0M BH 3 ·SMe 2 THF) (27.6 mL, 55.2 mmol), (2-methyl-4-nitro-phenyl)methanol (2a ), affording 4.62 g (˜quantitative yield) of the target compound (1a) as a pale yellow solid of sufficient purity to be used directly in the next step without further isolation and purification. R f : ~0.50 (EtOAc / Hxn=1:1, v / v). 1 H NMR (300MHz, CDCl 3):δ8.07(dd,J=8.4,2.1Hz,1H),8.02(d,J=2.1Hz,1H),7.62(d,J=8.1Hz,1H),4.79(s,2H),2.38 (s, 3H), 1.87 (br.s, 1H) ppm. Spectral data are consistent with those presented in the literature. The compounds are also commercially available.
[1482] Step B: 2-Methyl-4-nitro-benzaldehyde (2b)
[1483] Following the general procedure described in 2 (Variant B), in manganese dioxide (MnO 2 2-Methyl-4-nitro-be...
Embodiment 3
[1497] 3-Amino-4-[5-[bis(2-chloroethyl)amino]-2-methyl-phenyl]butanoic acid (3)
[1498]
[1499] Step A: 2-(Bromomethyl)-1-methyl-4-nitro-benzene (3a)
[1500] According to the general procedure described in 10, by using phosphorus tribromide (PBr 3 ) (1.0M PBr in DCM 3 ) solution (65.8 mL) bromination of (2-methyl-5-nitro-phenyl)methanol (1a) (11.0 g, 65.8 mmol) dissolved in dichloromethane (DCM) (110 mL) (as in Example 1) to prepare 2-(bromomethyl)-1-methyl-4-nitro-benzene (3a). Aqueous workup afforded 11.3 g (75% yield) of a pale yellow solid (3a) of sufficient purity to be used directly in the next step without further isolation and purification. R f : ~0.56 (EtOAc / Hxn=1:5, v / v). 1 H NMR (300MHz, CDCl 3 ): δ8.19(d, J=2.4Hz, 1H), 8.07(dd, J=8.4, 2.7Hz, 1H), 7.36(d, J=8.7Hz, 1H), 4.53(s, 2H), 2.52 (s,2H)ppm. Spectral data are consistent with those presented in the literature. The compounds are also commercially available.
[1501] Step B: Diethyl 2-acetylamino-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com